Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
There is a pressing need for novel biologic therapies in systemic lupus erythematosus (SLE). We sought to systematically review the outcomes of recent phase 3 clinical trials of biologic therapies for SLE and lupus nephritis, and conduct a meta-analysis of steroid sparing effect in these trials.
Methods:
A systematic review of the outcomes of phase 3 clinical trials of biologic therapies for SLE and lupus nephritis was undertaken. Studies were identified by searching the Medline (via Pubmed), EMBASE, CINAHL and SCOPUS databases, the Cochrane library, and clinicaltrials.gov. Adult human studies published in English in the last ten years (until 18 April 2017) were included. All articles that reported outcomes of phase 3, randomized, placebo-controlled clinical trials of biologic therapies for SLE or lupus nephritis were included. A random-effects meta-analysis to compare a common corticosteroid reduction endpoint in the trials of rituximab, belimumab, tabalumab and epratuzumab in SLE, was conducted.
Results:
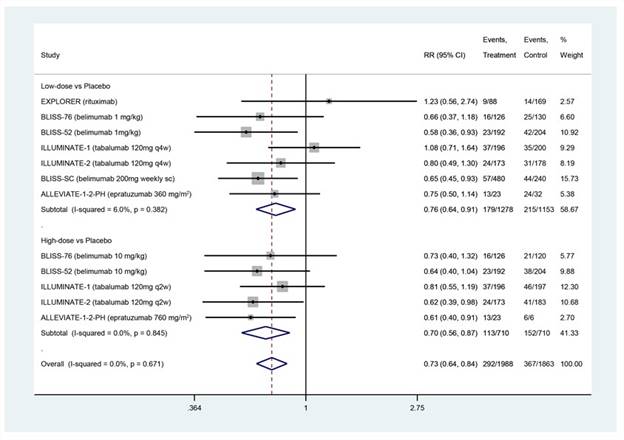
A total of twenty-eight studies were identified, with nine being conducted in SLE, five in lupus nephritis and the remaining fourteen being post hoc analyses of phase 3 trials in SLE. All biologic therapies trialed targeted B cells (rituximab, belimumab, tabalumab, epratuzumab, atacicept, ocrelizumab), except for abetimus sodium and abatacept. Only the three phase 3 trials of belimumab in SLE met their primary endpoints, although benefit in secondary endpoints and reduction in serological activity was often seen in the other studies. In particular, the meta-analysis (Figure 1) showed that most therapies (belimumab IV and SC, tabalumab and epratuzumab) had a steroid sparing effect, compared to placebo (pooled relative risk 0.76; 95% CI: 0.64, 0.91). Therapies were generally well tolerated, however, three studies were terminated prematurely due to serious side effects, and another due to discontinuation of drug supply. Several ongoing phase 3 trials of B cell and non-B cell agents are currently underway.
Figure 1. Meta-analysis of percentage of patients with decrease in corticosteroid dose from baseline:-
- to ≤ 7.5mg/day, by ≥ 25% from baseline between weeks 40-52 (BLISS-SC, BLISS-52, BLISS-76)
- to ≤ 7.5mg/day, between weeks 24-52 for ≥ 3 consecutive months without an increase in antimalarial or immunosuppressant therapy (ILLUMINATE-1 and -2)
- to ≤ 10mg/day or ≤ 7.5mg/day by week 24 (ALLEVIATE-1 and -2)
- < 10mg/day between week 24-52 in addition to a major clinical response (EXPLORER)
Conclusion:
Although almost all phase 3 clinical trials (with the exception of belimumab) have failed to meet their primary efficacy endpoint, meta-analysis shows a significant steroid sparing effect for novel biologic therapies in SLE. This highlights allowance of background steroid dose as a major methodologic consideration in the design of future SLE clinical trials.
To cite this abstract in AMA style:
Oon S, Huq M, Godfrey T, Nikpour M. Systematic Review, and Meta-Analysis of Steroid-Sparing Effect, of Biologic Agents in Randomized Placebo-Controlled Phase 3 Trials for Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/systematic-review-and-meta-analysis-of-steroid-sparing-effect-of-biologic-agents-in-randomized-placebo-controlled-phase-3-trials-for-systemic-lupus-erythematosus/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/systematic-review-and-meta-analysis-of-steroid-sparing-effect-of-biologic-agents-in-randomized-placebo-controlled-phase-3-trials-for-systemic-lupus-erythematosus/