Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Inflammatory bowel diseases (IBD) and anterior uveitis (AU) are frequent extra-articular features of axial spondyloarthritis (axSpA). Although effect of anti-TNF on IBD and AU are well known, data are lacking for anti-IL17A. Our objective was to assess and compare the incidence of IBD and AU in axSpA patients treated with anti-TNF and anti-IL17A.
Methods: A systematic literature review was performed by 2 independent reviewers in 3 databases (PubMed, EMBase and Cochrane) until 2019/04/04 and completed with 2016-18 ACR and EULAR abstracts. We included randomized controlled trials (RCT) assessing anti-TNF (monoclonal antibodies (Mab): adalimumab, certolizumab, golimumab, infliximab, and soluble receptor fusion protein: etanercept (ETN)) or anti-IL17A (ixekizumab, secukinumab) versus placebo or another biologic in axSpA according to ASAS criteria and reporting safety data on IBD or UA. History of IBD or AU was not an exclusion criterion in these RCTs, although recent onset or active IBD/AU was. The risk of bias in included RCTs was evaluated according to the Cochrane risk of bias tool. Data from these studies was used to perform a network meta-analysis to assess incidence of IBD and UA under each treatment, using the Mantel‐Haenszel method relevant for rare events (netmeta R Package).
Results: Out of initially 725 studies, 31 were included for analysis, provided a total of 3888 treated patients (anti-TNF MAb: 2011, ETN: 699, anti-IL17A: 1178) and 2207 placebo-receiving axSpA patients. The mean study duration was 20.8 weeks ± 17.4 (SD), median: 16 weeks. Incidence of AU was 1.09%, 2.14%, 3.53%, 3.26% per year in anti-TNF MAb, ETN, anti-IL17A and placebo groups, respectively. Incidence of IBD was 0.22%, 1.28%, 2.17%, 0.48% per year in anti-TNF MAb, ETN, anti-IL17A and placebo groups, respectively.
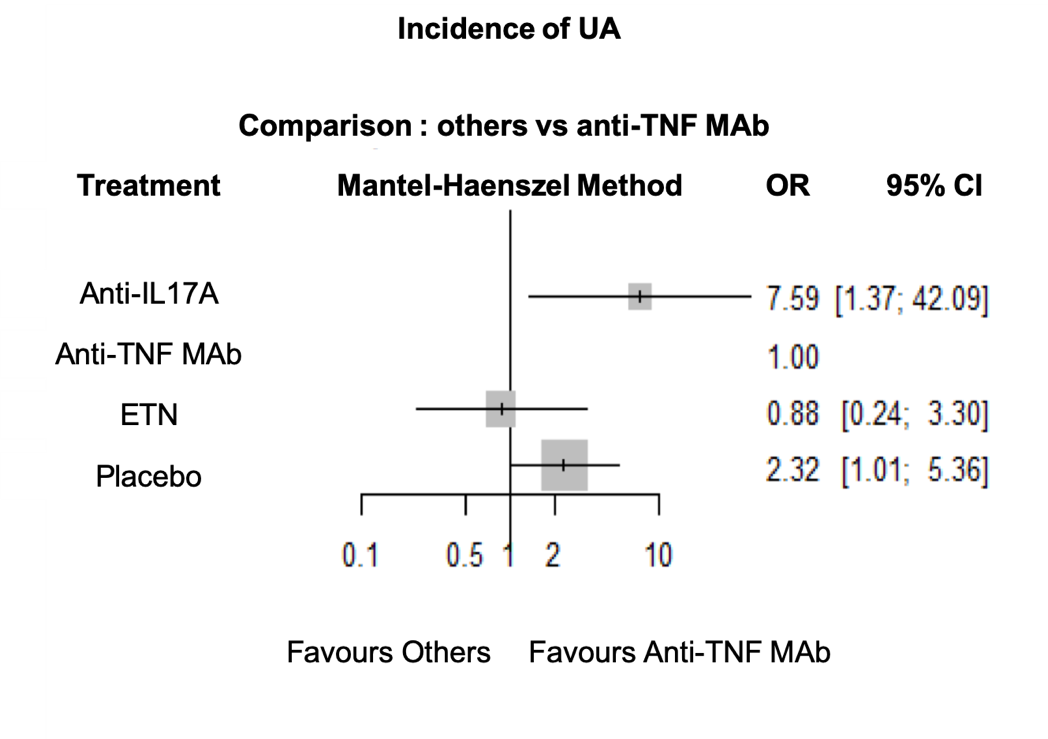
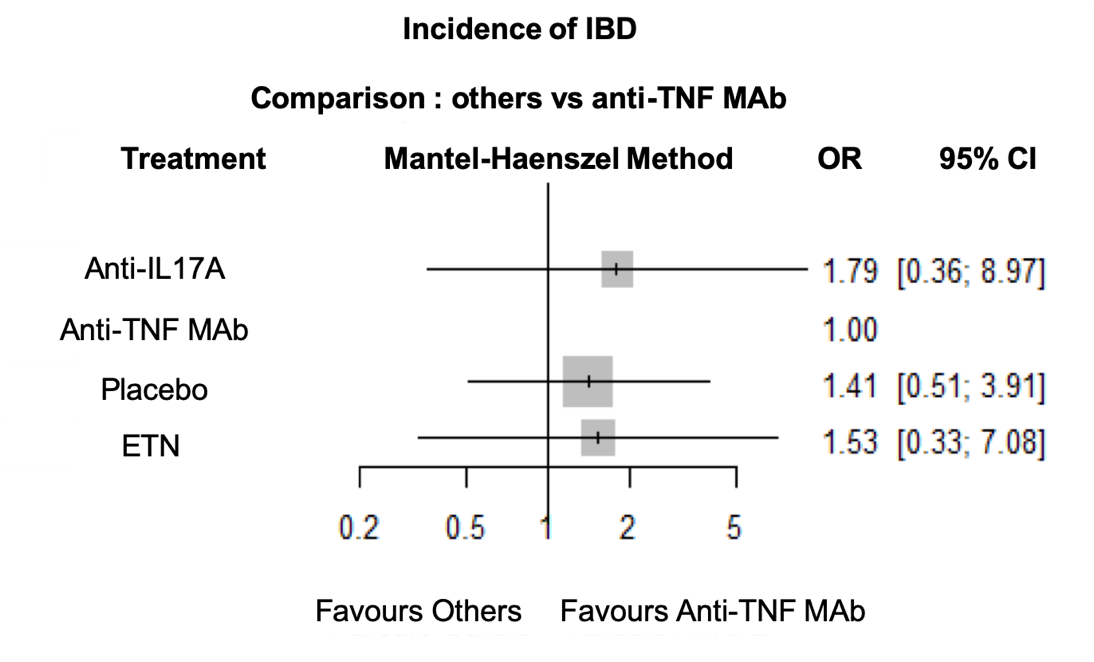
Incidence of UA was reduced with anti-TNF MAb compared to anti-IL17A (OR = 7.59; IC95% 1.37-42.09) and placebo (OR = 2.32; IC95% 1.01-5.36) (Figure 1). There was no statistical difference between anti-TNF MAb and ETN. There was no statistical difference in IBD incidence between anti-TNF MAb, ETN, anti-IL17A and placebo (Figure 2). No evidence of inconsistency was found in this network model.
Conclusion: In RCT assessing anti-TNF and anti-IL17A in axSpA, incident AU or IBD are rare events. However, this network meta-analysis demonstrate that anti-IL17A are associated with a higher incidence of AU compared to placebo and anti-TNF, while there was no statistical difference between treatments concerning IBD incidence.
To cite this abstract in AMA style:
Roche D, Badard M, Boyer L, Lafforgue P, Pham T. Systematic Literature Review and Network Meta-Analysis Comparing Incidence of Uveitis and IBD in Axial Spondyloarthritis Patients Treated with Anti-TNF versus Anti-IL17A in Placebo Controlled Randomized Trials [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/systematic-literature-review-and-network-meta-analysis-comparing-incidence-of-uveitis-and-ibd-in-axial-spondyloarthritis-patients-treated-with-anti-tnf-versus-anti-il17a-in-placebo-controlled-randomiz/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/systematic-literature-review-and-network-meta-analysis-comparing-incidence-of-uveitis-and-ibd-in-axial-spondyloarthritis-patients-treated-with-anti-tnf-versus-anti-il17a-in-placebo-controlled-randomiz/