Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose:
Chronic inflammatory diseases (CIDs) such as rheumatoid arthritis (RA), psoriatic arthritis (PsA), plaque psoriasis (PsO), ankylosing spondylitis (AS), Crohn’s disease (CD), and ulcerative colitis (UC) can be managed effectively with biologic agents such as originator infliximab (IFX). Although IFX biosimilars have been approved in the US since April 2016 for the treatment of CIDs, they have yet to be designated as interchangeable. The impact of switching patients stable on originator IFX to a biosimilar in the US is not well documented. This study compares switching patterns of patients stable on originator IFX switching to an IFX biosimilar (switchers) versus those remaining on originator IFX (continuers).
Methods:
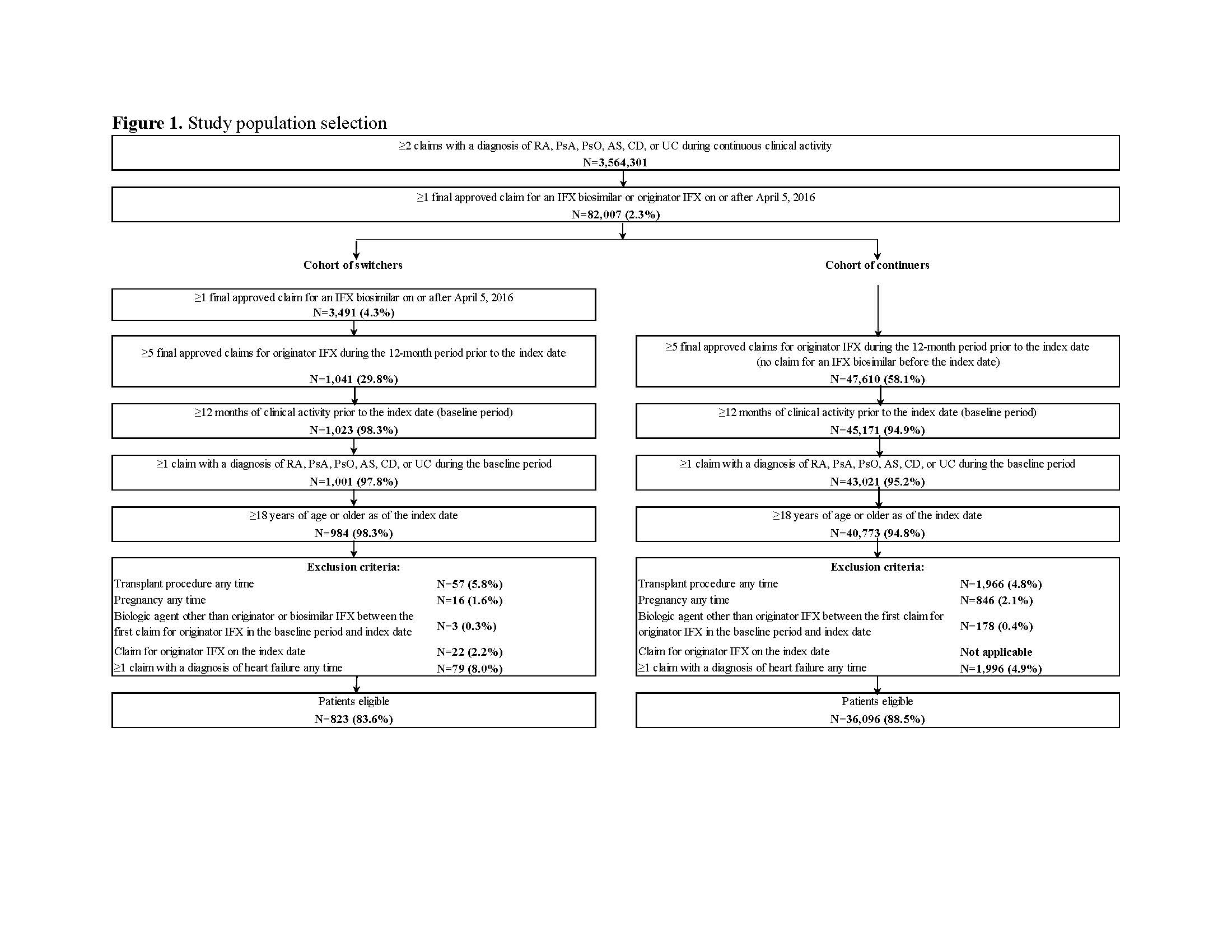
Symphony Health Solutions’ Patient Transactional Datasets, which includes comprehensive longitudinal US claims data, was used (10/2012–11/2018) to identify adults with ≥2 claims for either RA, PsA, PsO, AS, CD, or UC, and ≥1 claim for originator or biosimilar IFX (Figure 1). The index date (on or after 4/5/2016) was the first IFX biosimilar claim for switchers or a random originator IFX claim for continuers. Pre-index, patients had to be stable on originator IFX (i.e., ≥5 originator IFX claims in the 12 months pre-index). Switchers were matched 1:3 to continuers using propensity score matching adjusted for covariates such as age, gender, and type of CID (full list in Table 1). Post-index switching patterns were compared between switchers and continuers using hazard ratios (HRs), Kaplan Meier (KM) curves, and log-rank tests. Of note, the database didn’t include reasons for switching/discontinuation or prescriptions/services received outside of the network.
Results:
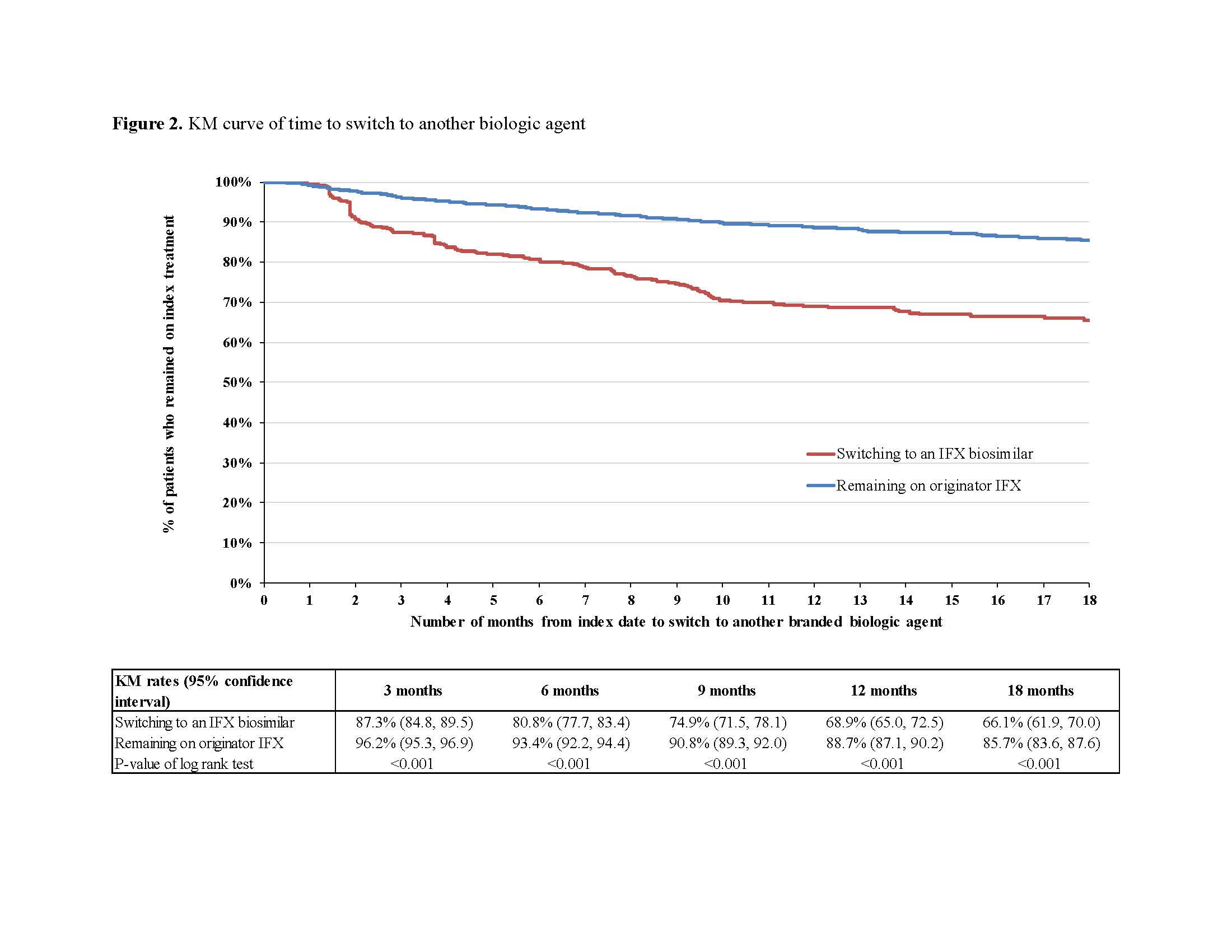
After matching, 823 switchers and 2,469 continuers were included. Among switchers, 44.3%, 33.9%, and 17.4% had RA, CD, and UC, respectively, versus 44.3%, 32.8%, and 18.3% for continuers. Mean age was 57.9 (standard deviation [SD]=16.2) and 57.1 (SD=16.3) years among switchers and continuers, respectively; 66.3% were female in both cohorts (Table 1). Over a mean post-index period length of 350 (SD=197) and 321 (SD=214) days for switchers and continuers, respectively, 211 (25.6%) switchers and 225 (9.1%) continuers switched to another branded biologic agent (HR=2.92; P< 0.001). Of 211 switchers, 82.5% switched back to originator IFX and 17.5% switched to another branded biologic agent. The mean time to switch to another branded biologic agent was 142 (SD=110) days for switchers and 174 (SD=142) days for continuers. The KM rates of switchers and continuers remaining on index treatment were 87.3% and 96.2% at 3 months post-index, 80.8% and 93.4% at 6 months, and 68.9% and 88.7% at 12 months, respectively (all log-rank P< 0.001; Figure 2).
Conclusion:
Patients with CIDs switching from originator to biosimilar IFX were almost 3 times more likely to switch to another branded biologic agent compared to patients remaining on originator IFX. Among those who switched to an IFX biosimilar and then to another branded biologic agent, >80% returned to originator IFX, on average within 5 months. Switching to IFX biosimilars should be carefully considered in light of the resulting risk of treatment instability and potential impact on clinical outcomes.
To cite this abstract in AMA style:
Emond B, Sadik K, Lafeuille M, Wynant W, Côté-Sergent A, Lefebvre P, Woodruff K, Fitzgerald T. Switching Patterns Among Patients with Chronic Inflammatory Diseases Switching to an Infliximab Biosimilar or Remaining on Originator Infliximab (REMICADE) [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/switching-patterns-among-patients-with-chronic-inflammatory-diseases-switching-to-an-infliximab-biosimilar-or-remaining-on-originator-infliximab-remicade/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/switching-patterns-among-patients-with-chronic-inflammatory-diseases-switching-to-an-infliximab-biosimilar-or-remaining-on-originator-infliximab-remicade/