Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: A surge in nocturnal inflammatory cytokines causing morning stiffness (MS) is recognized as a therapeutic target in RA. MS has been underappreciated in impacting the quality of life (QOL) of RA patients. Along with renewed interest in glucocorticoids (GCs) as an option for targeting these circadian disease fluctuations, ACR is updating its practice guidelines. Optimal timing for glucocorticoid administration will be a new and important topic to address. DR prednisone administered at bedtime produces significant absolute and relative reduction in morning symptoms. Inflammatory cytokines are also reduced significantly. In this analysis of patients with RA, we evaluated the reduction of symptoms that impact QOL. This assessment examined 9 months of patient reported diary data from the CAPRA I trial who were switched from IR-prednisone to DR-prednisone after 3 months of previous therapy.
Methods: RA patients previously randomized to IR prednisone (N=110) were switched to DR-prednisone open label and evaluated at 3, 6, and 9 months to measure median absolute (paired t-test) and relative changes (Wilcoxon Rank Sum Test) in pain, patient’s global, and MS as the primary outcome. Patients completed diary entries at least 7 days prior to each of the 3 month visits including waking time, stiffness (yes/no), time of resolution and intensity of pain (VAS) during the day. In an analysis not previously performed, all patient diary entries +/- 4 weeks of each visit were compared to Baseline at Switch in addition to other disease measures.
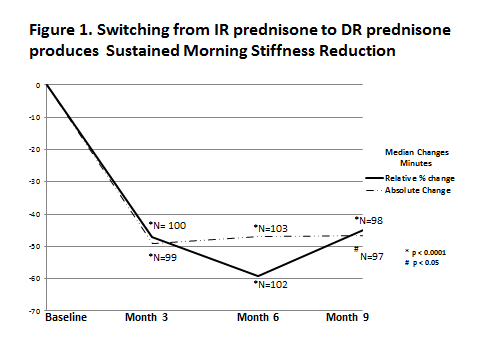
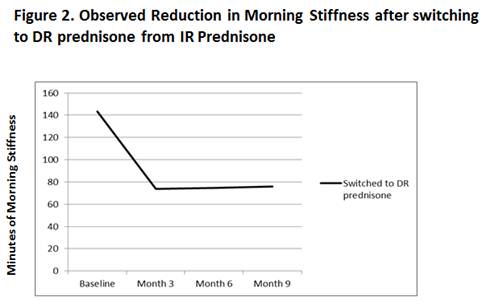
Results: There was a statistically significant and clinically meaningful reduction in morning stiffness during the first 3 months after the switch and the response was maintained over the entire study period of 9 months (Figure 1). Absolute reduction of MS was approximately 50 min from 143.5 min and there was a > 40% relative reduction in MS at each visit. IL-6 levels were reduced concomitantly by the same amount. The mean reduction in MS was over 60 minutes at each of the three visits (Figure 2).
Conclusion: This analysis demonstrates that RA patients on stable DMARD therapy, who have not adequately responded to IR-prednisone with respect to morning stiffness, showed statistically significant and clinically meaningful improvement in this symptom when switched to DR prednisone which markedly impacts quality of life. This beneficial effect was maintained over the 9 month study duration.
References:
ACR, Rheumatologist 2013;May
Buttgereit, et. al. Ann Rheum Dis 2010;69:127-1280
Alten, et. al. J Rheumatol 2010;37:2025-2031
Disclosure:
R. Alten,
Horizon Pharma, Inc,
5;
R. Holt,
Horizon Pharma, Inc,
5;
A. Grahn,
Horizon Pharma, Inc,
3;
P. Rice,
Horizon Pharma, Inc,
5;
J. Kent,
Horizon Pharma, Inc,
3;
F. Buttgereit,
Horizon Pharma, Inc,
2;
A. Gibofsky,
GlaxoSmithKline plc,
1,
Bristol-Myers Squibb,
1,
Johnson & Johnson,
1,
Horizon Phartmaceuticals,
5,
Iroko Pharmaceuticals,
5,
Abbott Laboratories,
9,
Amgen, Inc,
9,
Genentech, Inc,
9.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/switching-from-immediate-release-ir-prednisone-to-delayed-release-dr-prednisone-improves-patient-reported-outcomes-in-rheumatoid-arthritis-ra-patients-on-conventional-disease-modifying-antirheum/