Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: The treatment of rheumatoid arthritis (RA) has evolved continuously. The introduction of non-conventional DMARDs, which include biologic and targeted synthetic DMARDs (tsDMARDs), has been a breakthrough. Although there is plenty information on when to initiate these drugs, data on when to stop them is scant. The objective of this study was to assess the length of remission and relapse rate in patients with RA who had to discontinue tocilizumab (TCZ) or tsDMARDs (tofacitinib or baricitinib) due to the completion of extended long-term clinical trials, and to compare the survival curves of disease remission.
Methods: A prospective cohort study of RA patients in remission (DAS28 < 2.6, 0 swollen joints) assembled at the time of their last dose of either tocilizumab (subcutaneous [SC] or intravenous [IV]), tofacitinib or baricitinib. Patients were followed every 8 weeks through a COPCORD questionnaire and every 4 months by an in-office assessment. Doses of methotrexate were not changed. Patients were instructed to contact our center should any RA-related symptoms occur. The primary endpoint was relapse, defined as the presence of ≥1 swollen joints. Kaplan-Meier curves were calculated per type of drug (TCZ and tsDMARDs) and per route of administration (PO, SC and IV). A log-rank test was used to assess differences among survival curves and Cox regression was used to evaluate variables as predictors of relapse. Significance was set at p<0.05.
Results: Ninety-nine patients were analyzed, 88% female, with a mean age of 47±13 years. Sixty patients were treated with TCZ-IV, 18 with TCZ-SC and 21 with tsDMARDs. During the follow-up of 165 person-years, 27 patients (27%) maintained remission. The median time until relapse was 9 months. No significant differences were found between the type of drug or the administration route. No variables were identified as relapse predictors. 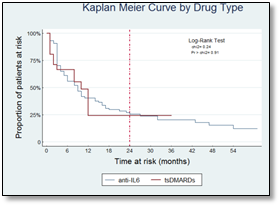

Conclusion: Long-term remission is possible in a proportion of patients after suspending a non-conventional DMARD. This effect has been previously reported with multiple biologic agents but not with tsDMARDs. Further studies are required to establish algorithms on the discontinuation of non-conventional DMARDs after achieving remission.
To cite this abstract in AMA style:
Ramirez-Gomez A, Barajas-Ochoa A, Castaneda-Sanchez JJ, Castillo-Ortiz JD, Sanchez-Gonzalez JM, Ramos-Remus C. Sustained Remission and Relapse Rate after Non-Conventional DMARD Withdrawal in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/sustained-remission-and-relapse-rate-after-non-conventional-dmard-withdrawal-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustained-remission-and-relapse-rate-after-non-conventional-dmard-withdrawal-in-patients-with-rheumatoid-arthritis/
