Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
Axial spondyloarthritis (axSpA) includes both ankylosing spondylitis (AS, meeting modified New York criteria) and axSpA with no sacroiliitis on X-ray (non-radiographic axSpA, nr-axSpA). Previous results show significant improvements in workplace and household productivity and social participation with certolizumab pegol (CZP) vs placebo (PBO) up to Week (Wk) 24 in the RAPID-axSpA study, which were continued to Wk48.1 This report investigates the long-term effect of CZP on workplace and household productivity up to 96 wks in patients (pts) with axSpA, including AS and nr-axSpA.
Methods
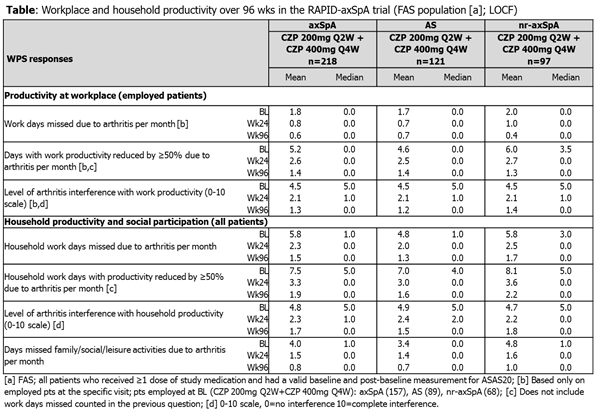
The ongoing RAPID-axSpA trial (NCT01087762),2 a 204-wk Phase 3 study in axSpA pts, is double-blind and PBO-controlled to Wk24, dose-blind to Wk48 and open-label to Wk204. Pts had active axSpA, according to ASAS criteria,3 including both AS and nr-axSpA pts. Pts originally randomized to CZP (200mg Q2W or 400mg Q4W, following 400mg loading dose [LD] at Wks 0, 2, 4) continued on their assigned dose in the OLE; PBO pts entering dose-blind phase were re-randomized to CZP LD followed by CZP 200mg Q2W or CZP 400mg Q4W after Wk24 or, for non-responders, after Wk16. The validated arthritis-specific Work Productivity Survey (WPS; administered Q4W)4 assessed the impact of axSpA on workplace and household productivity. WPS responses (LOCF imputation) in pts originally randomized to CZP are summarized descriptively over 96 wks.
Results
A total of 325 pts were randomized, of whom 218 received CZP (200mg Q2W or 400mg Q4W) from Wk0. Of the pts randomized to CZP at baseline (BL), 93% completed Wk24, 88% Wk48 and 80% Wk96. At BL, 72% of CZP pts were employed outside of the home. Pts randomized to CZP reported ~1 wk of paid work, ~2 wks of household duties, and mean 4.0 days of social activities affected over previous month. Employed pts in both CZP groups reported reductions in workplace absenteeism and presenteeism to Wk24, with continued improvements to Wk96 (Table). CZP pts reported continued improvements in household productivity and social participation up to Wk96 (Table). Similar improvements were observed in AS and nr-axSpA pts (Table).
Conclusion
The initial improvements with CZP in workplace and household productivity and increased participation in social/leisure activities were continued to Wk96 in axSpA, AS and nr-axSpA pts.
References
1. van der Heijde D. Arthritis Rheum 2013;65(10):1520
2. Landewé R. Ann Rheum Dis 2014;73:39-47
3. Rudwaleit M. Ann Rheum Dis 2009;68(6):770-776
4. Osterhaus J. Arth Res Ther 2014 [in press]
Disclosure:
D. van der Heijde,
AbbVie, Amgen, AstraZeneca, Augurex, BMS, Celgene, Centocor, Chugai, Covagen, Daiichi, Eli-Lilly, Galapagos, GSK, Janssen Biologics, Merck, Novartis, Novo-Nordisk, Otsuka, Pfizer, Roche, Sanofi-Aventis, Schering-Plough, UCB, Vertex,
5,
Imaging Rheumatology bv,
9;
J. Braun,
Abbott, Bristol Myers Squibb, Celgene, Celltrion, Chugai, Johnson & Johnson, MSD, Novartis, Pfizer, Roche, UCB Pharma,
5,
Abbott, Bristol Myers Squibb, Celgene, Celltrion, Chugai, Johnson & Johnson, MSD, Novartis, Pfizer, Roche, UCB Pharma,
2;
M. Rudwaleit,
Abbott, BMS, MSD, Pfizer, Roche, UCB Pharma. ,
5;
O. Purcaru,
UCB Pharma,
3;
A. Kavanaugh,
Abbott, Amgen, BMS, Pfizer, Roche, Janssen, UCB Pharma,
2.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sustained-improvements-in-workplace-and-household-productivity-and-social-participation-with-certolizumab-pegol-over-96-weeks-in-patients-with-axial-spondyloarthritis-including-ankylosing-spondylitis/