Session Information
Date: Monday, November 11, 2019
Title: 4M114: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science (1872–1877)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: There are limited data on patients transitioning from denosumab (DMAb) to bisphosphonates (BPs). The Denosumab Adherence Preference Satisfaction (DAPS) study (NCT00518531) reported that alendronate (ALN) could maintain the gains in bone mineral density (BMD) achieved with 1 year of DMAb treatment (Freemantle Osteoporos Int 2012). Here, we investigate relationships between subject characteristics and their BMD responses after transitioning from DMAb to ALN.
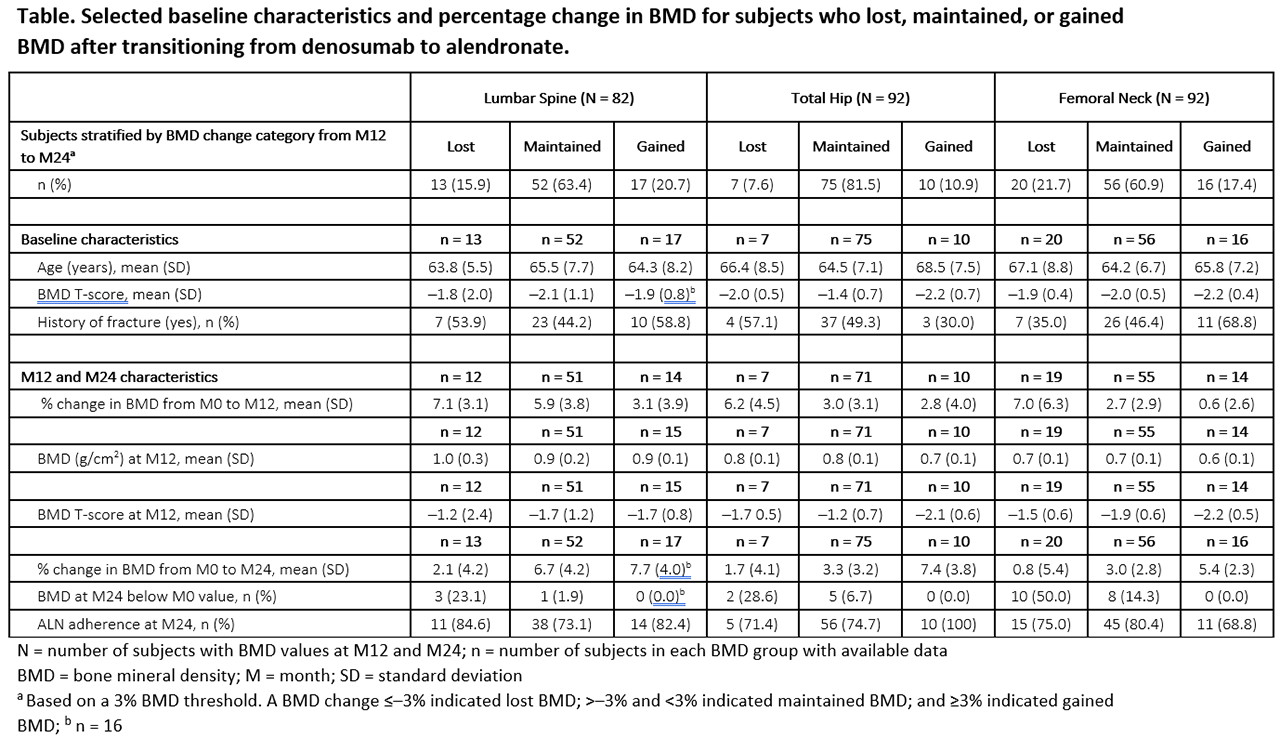
Methods: DAPS was a 24-month, open-label, randomized, cross-over study designed to compare adherence to 12 months of DMAb treatment (60 mg Q6M SC) with ALN (70 mg QW PO) in treatment-naïve postmenopausal women with a T-score ≤─2.0 to ≥─4.0 at the lumbar spine (LS), total hip (TH), or femoral neck (FN). BMD was measured at baseline and months (M) 12 and 24. Here we evaluate subjects transitioning from DMAb to ALN at M12. A 3% BMD least significant change threshold identified subjects who lost, maintained, or gained BMD from M12 to M24 (change ≤─3%, >─3% and < 3%, or ≥3%, respectively); baseline, M12, and M24 characteristics were summarized using descriptive statistics.
Results: Of 126 subjects randomized to DMAb, 115 (91%) transitioned to ALN at M12. At baseline, subjects had a mean age of 65 years and mean BMD T-scores of ─2.0, ─1.6, and –2.0 at the LS, TH, and FN, respectively. BMD increased by 5.6%, 3.2%, and 3.1% with DMAb from M0 to M12 at the LS, TH, and FN, respectively, and changed by 0.6%, 0.4%, and –0.1% with ALN from M12 to M24. After transitioning from DMAb to ALN, most subjects showed maintained or increased BMD (Table); 15.9%, 7.6%, and 21.7% lost BMD at the LS, TH, and FN, respectively, and only 1 subject (1.2%) lost BMD at all 3 sites. Baseline characteristics, M12 BMD, and adherence to oral ALN showed no trend with the BMD change from M12 to M24. However, subjects who lost BMD from M12 to M24 on ALN showed greater gains in BMD from M0 to M12 on DMAb, and few who lost BMD fell below their pre-study baseline BMD value. No subject experienced clinical vertebral fracture.
Conclusion: ALN can effectively maintain the BMD gains accrued after 1 year of DMAb in most subjects. Those with larger BMD increases in year 1 are more likely to lose BMD in year 2, with other subject characteristics not predictive of the response in year 2. These data highlight the need for oral BP therapy following DMAb cessation and BMD monitoring of patients transitioning from DMAb to ALN.
To cite this abstract in AMA style:
Kendler D, Chines A, Clark P, Ebeling P, McClung M, Rhee Y, Huang S, Kees Stad R, Freemantle N. Subject Characteristics and Changes in Bone Mineral Density After Transitioning from Denosumab to Alendronate in the Denosumab Adherence Preference Satisfaction (DAPS) Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/subject-characteristics-and-changes-in-bone-mineral-density-after-transitioning-from-denosumab-to-alendronate-in-the-denosumab-adherence-preference-satisfaction-daps-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/subject-characteristics-and-changes-in-bone-mineral-density-after-transitioning-from-denosumab-to-alendronate-in-the-denosumab-adherence-preference-satisfaction-daps-study/