Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: In EU, abatacept (ABA) with MTX is approved in patients (pts) with polyarticular-course JIA (pJIA), as young as 2 years (SC) and 6 years (IV), intolerant or with an inadequate response to prior DMARDs, and can be used as monotherapy if MTX use is inappropriate.1 In US, ABA can be used in combination with MTX or as monotherapy.2 Here we report ABA efficacy and safety in pts without concomitant MTX or in pts with prior biologic use treated in an open-label, Phase III study of SC ABA (NCT01844518) and in a double-blind, Phase III study of IV ABA (NCT00095173).
Methods: Study design and eligibility criteria for both studies were reported previously.3,4 In SC study, pts with pJIA and prior DMARD failure/intolerance were stratified by age cohort (2–5 and 6–17 years) to receive weight-tiered SC ABA (10 to < 25 kg [50 mg], 25 to < 50 kg [87.5 mg], ≥50 kg [125 mg]) weekly for 4 months (primary endpoint). JIA-ACR30 criteria responders at Month 4 could receive SC ABA for another 20 months.3 In IV study, pts with pJIA and prior DMARD failure/intolerance received open-label IV ABA (10 mg/kg body weight) for 4 months (Period A); JIA-ACR30 criteria responders at Month 4 were randomized 1:1 to receive IV ABA (10 mg/kg body weight) or placebo every 4 weeks for 6 months or until flare (Period B). Pts could receive open-label ABA in a 5-year follow-up (Period C).4 In this analysis, data were evaluated descriptively.
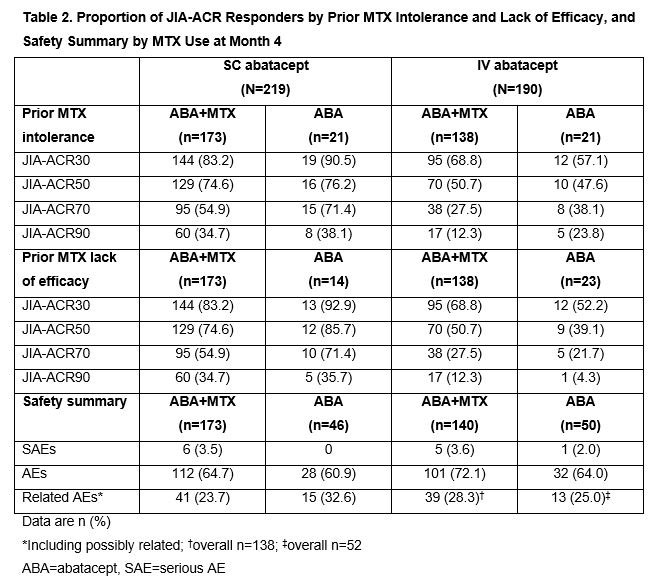
Results: Baseline characteristics were mostly balanced between the arms in both studies (Table 1). No additional numerical benefit in JIA-ACR responses was seen with ABA+MTX versus ABA monotherapy in either study at Month 4 (JIA-ACR90: SC ABA+MTX, 34.7% [60/173] vs SC ABA, 34.8% [16/46]; IV ABA+MTX, 12.3% [17/138] vs IV ABA, 13.5% [7/52]). Flare rates (95% CI) were comparable with ABA+MTX and ABA monotherapy in Period B of the IV study: 18.8% (7.7, 29.8) and 25.0% (0.5, 49.5), respectively. Efficacy responses of ABA+MTX and ABA monotherapy-treated JIA-ACR responders with prior MTX intolerance/lack of efficacy at Month 4 were similar in both studies (Table 2). As expected, ABA-treated biologic-naïve pts had a numerically greater clinical response than those with prior biologic use at Month 4 in both trials (Table 3); effect was independent of concomitant MTX use (data not shown). Rates of reported AEs were comparable for ABA+MTX- versus ABA-treated pts and for biologic-naïve versus those with prior biologic use across studies over 4 months (Tables 2, 3). No anti-drug antibodies (ADAs) were reported with ABA monotherapy in SC study; few pts receiving ABA monotherapy in IV study developed ADAs, with no impact on ABA efficacy seen (data not shown).
Conclusion: Abatacept (SC and IV) monotherapy was effective and well tolerated in pts with pJIA intolerant to MTX or when prior MTX was not effective. In addition, abatacept monotherapy can be considered for use in those with prior biologic therapy if MTX use is inappropriate.
References:
- Orencia [SmPC]. Princeton, NJ: Bristol-Myers Squibb; 2019.
- Orencia [prescribing information]. Princeton, NJ: Bristol-Myers Squibb; 2019.
- Brunner HI, et al. Arthritis Rheumatol 2018;70:1144–54.
- Ruperto N, et al. Lancet 2008;372:383–91.
Writing support: Katerina Kumpan, Caudex, funded by Bristol-Myers Squibb
To cite this abstract in AMA style:
Ruperto N, Lovell D, Bohnsack J, Breedt J, Fischbach M, Lutz T, Minden K, Miraval T, Ally M, Rubio-Pérez N, Gervais E, van Zyl R, Wong R, Nys M, Martini A, Brunner H. Subcutaneous or Intravenous Abatacept Monotherapy in Pediatric Patients with Polyarticular-Course JIA: Results from Two Phase III Trials [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/subcutaneous-or-intravenous-abatacept-monotherapy-in-pediatric-patients-with-polyarticular-course-jia-results-from-two-phase-iii-trials/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/subcutaneous-or-intravenous-abatacept-monotherapy-in-pediatric-patients-with-polyarticular-course-jia-results-from-two-phase-iii-trials/