Session Information
Date: Tuesday, October 23, 2018
Title: Pediatric Rheumatology – Clinical Poster III: Juvenile Idiopathic Arthritis and Uveitis
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
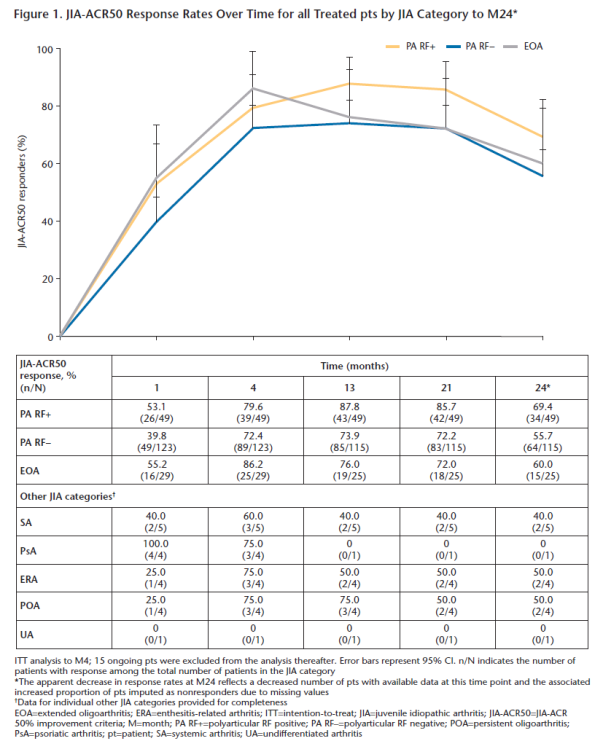
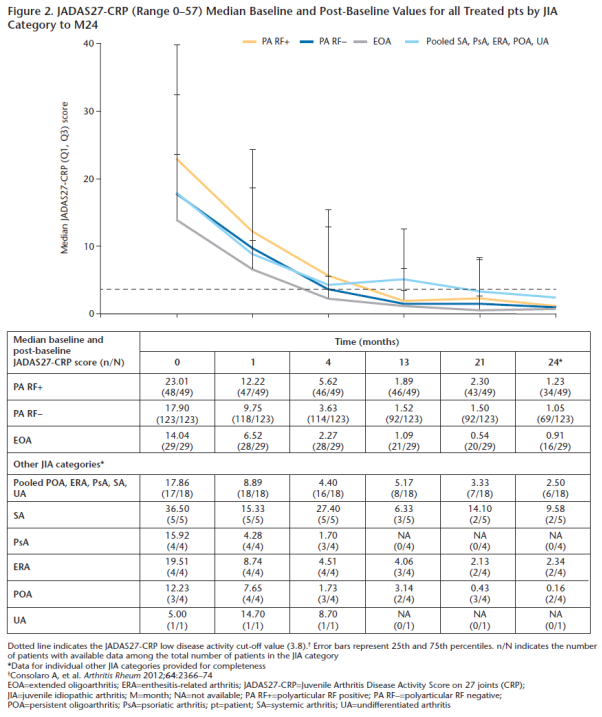
Conclusion: In this analysis of pts aged 2–17 yrs with JIA, SC abatacept was effective and well tolerated and improved HRQoL across JIA categories over 24M.
References:
To cite this abstract in AMA style:
Ruperto N, Brunner HI, Vega-Cornejo G, Berman A, Cuttica RJ, Ávila-Zapata F, Henrickson M, Kingsbury DJ, Bohnsack JF, Lutz T, Rubio-Pérez NE, Gerloni V, Li X, Nys M, Wong R, Martini A, Lovell DJ. Subcutaneous Abatacept in Patients Aged 2–17 Years with Juvenile Idiopathic Arthritis and Inadequate Response to Biologic or Non-Biologic Disease-Modifying Antirheumatic Drugs: Results over 24 Months By Juvenile Idiopathic Arthritis Disease Category [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/subcutaneous-abatacept-in-patients-aged-2-17-years-with-juvenile-idiopathic-arthritis-and-inadequate-response-to-biologic-or-non-biologic-disease-modifying-antirheumatic-drugs-results-over-24/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/subcutaneous-abatacept-in-patients-aged-2-17-years-with-juvenile-idiopathic-arthritis-and-inadequate-response-to-biologic-or-non-biologic-disease-modifying-antirheumatic-drugs-results-over-24/
