Session Information
Date: Tuesday, October 23, 2018
Title: Osteoporosis and Metabolic Bone Disease – Basic and Clinical Science Poster
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Spontaneous Vertebral Fractures after Denosumab Discontinuation.
H. Florez, J. Ramírez, A. Monegal, N. Guañabens, P. Peris.
Department of Rheumatology. Hospital Clinic, University of Barcelona.
Background/Purpose:
Denosumab (Dmab) is an antiresorptive treatment with demonstrated efficacy in osteoporosis. However, discontinuation of Dmab has been associated with rapid bone loss, and recently, the development of vertebral fractures (VF) in some patients. It is essential to identify the risk factors for these adverse events and follow its evolution.
The objective of this study is to analyse the clinical characteristics, parameters of bone metabolism and evolution of patients developing VF after Dmab discontinuation.
Methods:
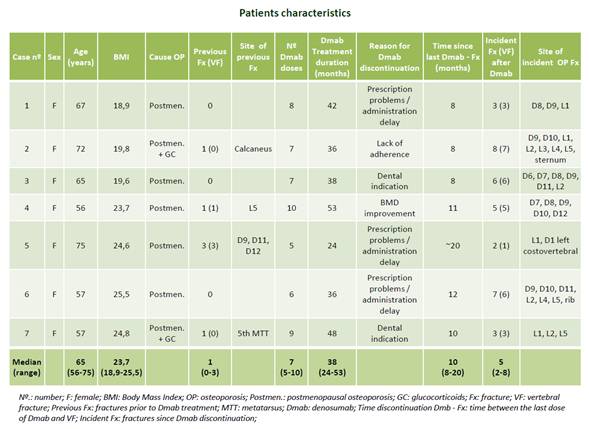
Seven women with spontaneous VF after Dmab discontinuation were included (median age 64 years [56-75]). The clinical history, cause of osteoporosis, treatments received, fractures, Dmab treatment duration and discontinuation period were reviewed. Additionally, the clinical and densitometric evolution, and bone mineral parameters were also analysed after Dmab discontinuation.
Results:
All the patients had postmenopausal osteoporosis, and two were receiving glucocorticoid treatment; 4/7 patients had previous fractures (2 VF, 1 calcaneus and 1 MTT); 5/7 had previously received antiosteoporotic treatment (hormone replacement therapy, risedronate, alendronate, zoledronate [once or consecutively)] during 6 months – 23 years. All had received Dmab for 24-53 months (median 38). The reasons for treatment discontinuation were: dental indication (2 patients), BMD improvement (T-score -1.2) (1 patient), poor adherence (1), prescription problems and/or delay in administration (3). The median bone mineral density T-scores prior to VF were -2.45 (-1.2/-4) at the lumbar spine and -2.1 (-0.6/-3.1) at the femoral neck. The mean time between the last Dmab dose and VF was 10 months (8-20), with a median of 5 VFs/patient (2-8). No patient showed 25-OH vitamin D <20 ng/ml. After Dmab discontinuation, bone turnover markers increased (median increase +364% in PINP and +287% in NTx); one patient presented hypercalcaemia (calcium 11.3mg/dL); and BMD decreased 1-21% in the lumbar spine and 2-6% in total hip at 8-22 months. After VF, 3 patients restarted Dmab, 1 Dmab+Teriparatide, 1 received zoledronate and 2 alendronate. No new fractures occurred during follow-up.
Conclusion:
Discontinuation of Dmab is associated with an increase in bone turnover markers and bone loss which can be associated with the development of spontaneous VF. Previous bisphosphonate therapy does not seem to decrease this risk. Further studies are needed to assess the optimal antiresorptive treatment and its duration after Dmab discontinuation.
To cite this abstract in AMA style:
Florez H, Ramírez J, Monegal A, Guañabens N, Peris P. Spontaneous Vertebral Fractures after Denosumab Discontinuation [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/spontaneous-vertebral-fractures-after-denosumab-discontinuation/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spontaneous-vertebral-fractures-after-denosumab-discontinuation/