Session Information
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: In the United States (US), the diagnosis of axial spondyloarthritis (axSpA) continues to be delayed by an average of 10 years from symptom onset. This diagnostic delay prevents effective management that controls disease activity, and maintains mobility, function, and quality of life. We developed the first SPARTAN recommendations for the referral of adults with chronic back pain to a rheumatologist for evaluation of axSpA by convening a multidisciplinary group comprised of clinicians that manage patients with back pain and conditions related to axSpA, expert rheumatologists and axSpA patient partners.
Methods: A systematic literature review (SLR) was conducted including studies through March 2022 to address individual clinical, laboratory, and imaging features associated with diagnosis or classification of axSpA. Sensitivity, specificity, positive likelihood ratios (LR+), and positive predictive values for each axSpA feature were calculated. At the 2022 SPARTAN annual meeting, members were asked the minimal probability of axSpA diagnosis that was appropriate for an adult with chronic back pain to be referred to a rheumatologist. In a Delphi exercise, members were asked to review test characteristics for each axSpA feature and to vote whether to include or exclude that feature in draft referral recommendations. Features gaining 70% consensus for inclusion were carried forward while those with consensus to exclude were omitted. Features that did not gain consensus for inclusion/exclusion were evaluated using discrete choice experiments (DCE). LR+ of individual axSpA features were used to calculate the probability of axSpA for combinations of features to develop draft referral recommendation for expert and stakeholder consideration. Draft recommendations in the form of a ‘major and minor criteria’, and a ‘points-based’ system were discussed and put to vote by SPARTAN members at the 2023 annual SPARTAN meeting.
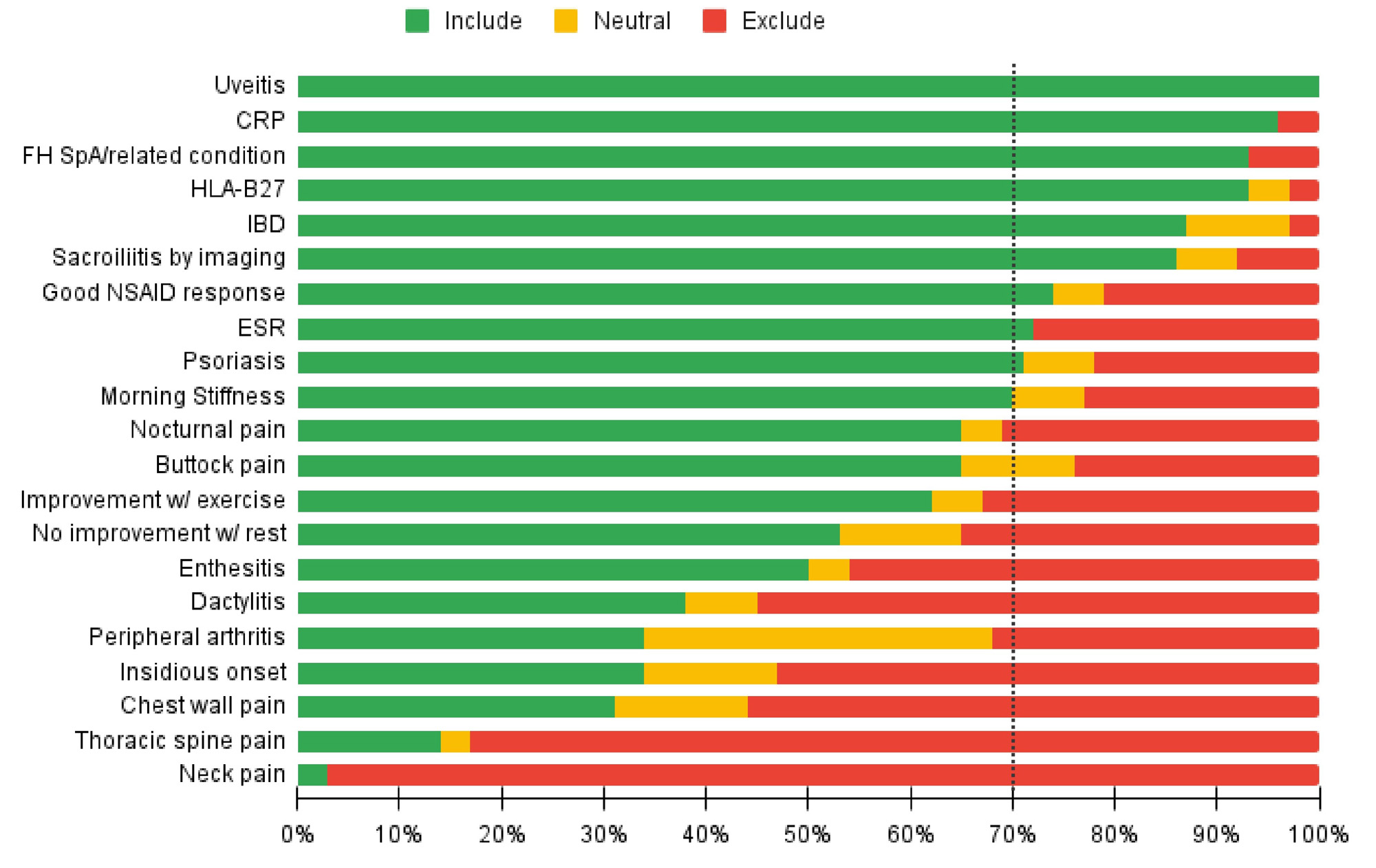
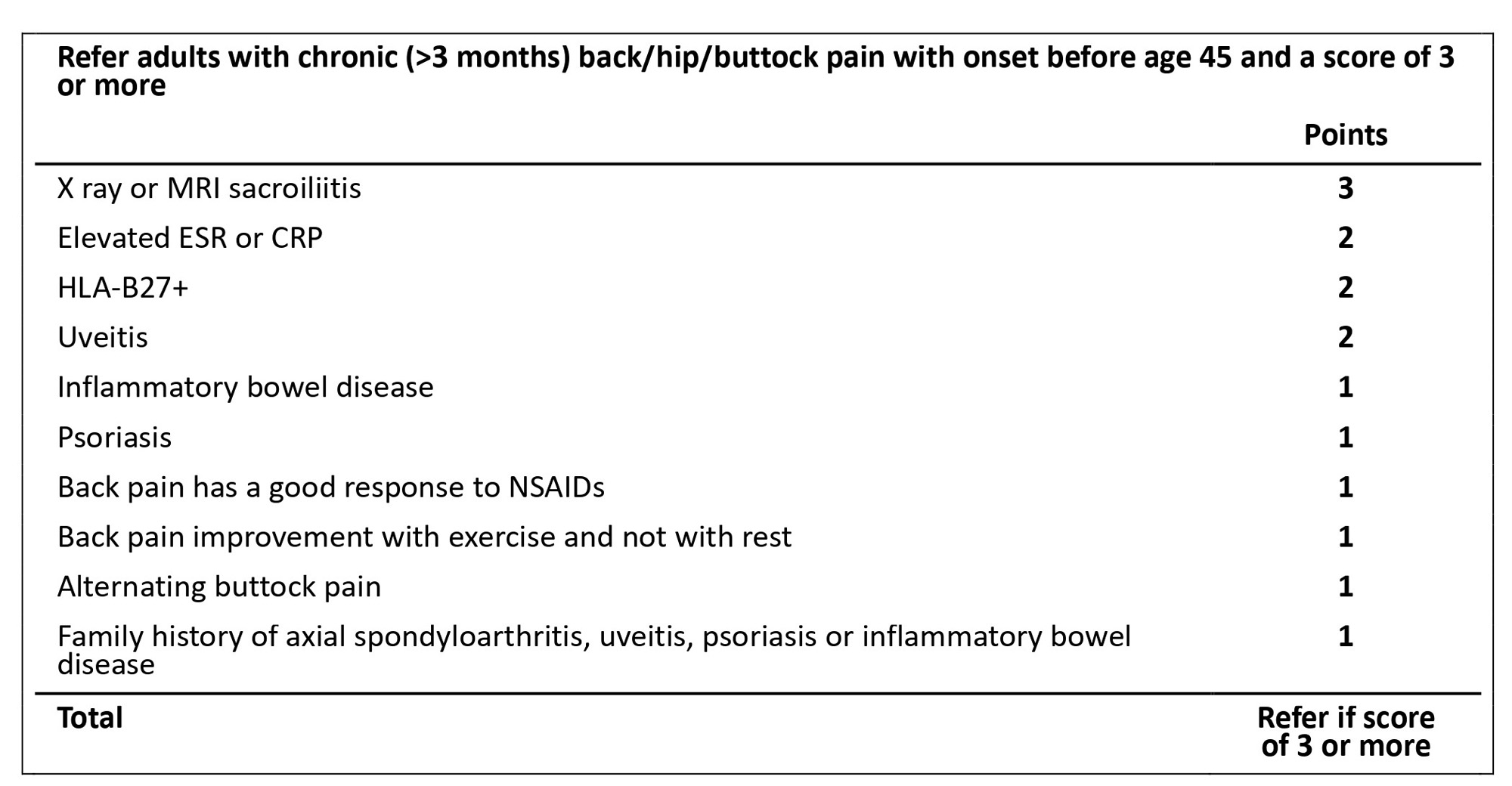
Results: SLR uncovered 28 features associated with axSpA. LR+ ranged from 0.5 to 10. A probability of 33% or higher was considered adequate for referral by 90% of SPARTAN members. The Delphi process resulted in consensus to include: uveitis, elevated ESR or CRP, family history of SpA or a related condition, HLA-B27 positivity, inflammatory bowel disease (IBD), sacroiliitis by imaging, good response to NSAIDs and psoriasis (Figure 1). There was consensus to exclude neck pain and thoracic spine pain. DCE-derived relative importance values of several features were approximately half as important as uveitis. Eighty-six percent of SPARTAN members preferred a point-based referral strategy and 89% voted in favor of its adoption (Figure 2).
Conclusion: SPARTAN members and a diverse stakeholder group used a data-driven process to develop the first draft SPARTAN recommendations for referral of adults with chronic back pain to a rheumatologist for evaluation of axSpA. Validation is needed to determine if application of these recommendations leads to approximately 33% probability of axSpA among those referred, and ultimately whether diagnostic delay of axSpA is reduced through their implementation.
To cite this abstract in AMA style:
Dubreuil M, Danve A, Alexander S, Bittar M, Fraenkel L, Grimshaw A, Kumthekar A, LaValley M, liew J, Magrey M, Majithia V, Merjanah S, Norton H, Walsh J, Deodhar A. Spondyloarthritis Research and Treatment Network (SPARTAN) Draft Referral Recommendations for Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/spondyloarthritis-research-and-treatment-network-spartan-draft-referral-recommendations-for-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spondyloarthritis-research-and-treatment-network-spartan-draft-referral-recommendations-for-axial-spondyloarthritis/