Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: By using low dose computed tomography (ldCT) in patients with radiographic axial spondyloarthritis (r-axSpA) we have recently shown that there is substantial progression of new bone formation in the facet joints within two years. We now aimed to assess the added value of scoring progression of whole spine facet joint ankylosis in addition to whole spine syndesmophytes in the quantification of spinal bone formation by ldCT.
Methods: In an observational cohort, patients with r-axSpA (≥1 inflammatory lesion on spinal MRI and 1-18 syndesmophytes in cervical and lumbar spine on conventional radiography) underwent whole spine ldCT (circa 4 mSv) at baseline and after 2 years. Two trained readers independently assessed paired ldCTs, blinded to chronology. Left and right facet joints from C2-S1, with the exception of C5-T2 due to low visibility, were scored as ankylosis present (1) or absent (0). Syndesmophytes were scored on a scale 0-552 according the Computed Tomography Syndesmophyte Score (CTSS)[1]. Average scores of the readers were used. Patients were included if at baseline they had < 80% ankylosed facet joints and < 80% bridged syndesmophytes on ldCT and had scores from both readers for both timepoints. Change scores are calculated as the total change in the whole spine per patient. The proportion of patients with a change score >0.5 and >SDC are presented and in addition net progression, defined as number of patients with change >0.5 minus number of patients with change < -0.5 divided by the total number of patients. Percentage agreement between progression in facet ankylosis and syndesmophyte formation is presented and a cumulative probability plot is shown.
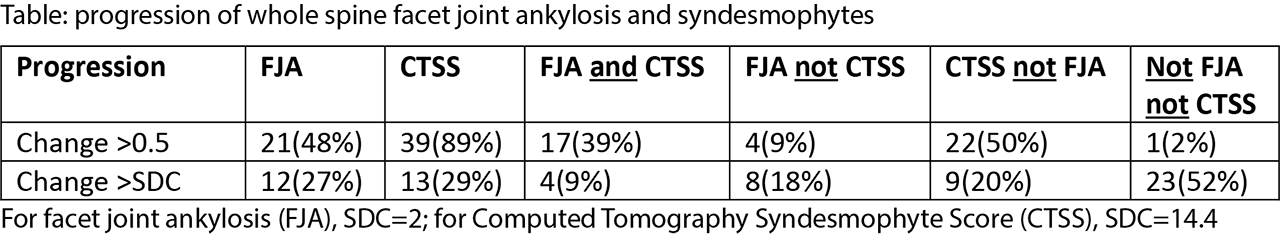
Results: A total of 44 patients was included in the analysis (mean age 49.4, 84% male, 79% HLA-B27+). At the group level, net progression of facet joint ankylosis was 43% (19/44) and net progression of syndesmophytes was 82% (36/44). The proportion of patients with progression is given in the Table. The percentage of patients with progression of facet joint ankylosis >0.5 was 48% and with syndesmophyte progression >0.5 was 89%. Using a more conservative cut-off for progression ( >SDC), the percentage of patients with progression of facet joint ankylosis was 27% and syndesmophyte progression was seen in 29%. Bone formation in the facet joints only occurred in 9% and 18% of the patients, and syndesmophytes progression only in 50% and 20% of the patients for the cut-offs of 0.5 and SDC, respectively. The figure shows that there is a tendency that patients with more facet joint ankylosis progression also had more syndesmophyte progression. However, in a number of patients these processes occurred independently.
Conclusion: These data show that scoring facet joint ankylosis in addition to syndesmophyte formation is useful to get full insight in the progression of spinal bone formation in patients with r-axSpA.
[1] de Bruin, F., et al. (2018). “Development of the CT Syndesmophyte Score (CTSS) in patients with ankylosing spondylitis: data from the SIAS cohort.” Annals of the rheumatic diseases 77(3): 371-377
To cite this abstract in AMA style:
Stal R, van Gaalen F, Sepriano A, Braun J, Reijnierse M, van der Heijde D, Baraliakos X. Spinal Bone Formation as Assessed by Low-Dose CT Scan in Patients with Radiographic Axial Spondyloarthritis – Comparison of the Progression Observed in Vertebrae and Facet Joints [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/spinal-bone-formation-as-assessed-by-low-dose-ct-scan-in-patients-with-radiographic-axial-spondyloarthritis-comparison-of-the-progression-observed-in-vertebrae-and-facet-joints/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spinal-bone-formation-as-assessed-by-low-dose-ct-scan-in-patients-with-radiographic-axial-spondyloarthritis-comparison-of-the-progression-observed-in-vertebrae-and-facet-joints/