Session Information
Date: Saturday, November 12, 2022
Title: SLE – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: To describe demographic characteristics, efficacy and safety of BLM since its approval in SLE patients in a real-world setting.
Methods: Descriptive, retrospective, multicenter study that analyze patients diagnosed with SLE according to EULAR/ACR 2019, SLICC and/or ACR 1997 diagnostic criteria. Data were collected from medical records of SLE patients treated with BLM since 2011. Demographic features and administration routes of BLM were assessed.
To evaluate efficacy, analytical variables (anti-DNA, C3, C4), SLE Disease Activity Index (SLEDAI) and steroid dose at baseline 3, 6, 12, 24, 36, 48 and 60 months were analyzed. Inefficacy was reported as no having improvement in clinical and/or analytical sets. To see whether a trend in BLM prescription had changed or not over time, two periods of time were analyzed: 2011-2016 (period1) and 2017-2022 (period2).
Renal improvement was evaluated as having a 24 hour-proteinuria < 0.5 g/day.
Safety was reported as adverse events incidence, infections and/or tumors leading to treatment discontinuation.
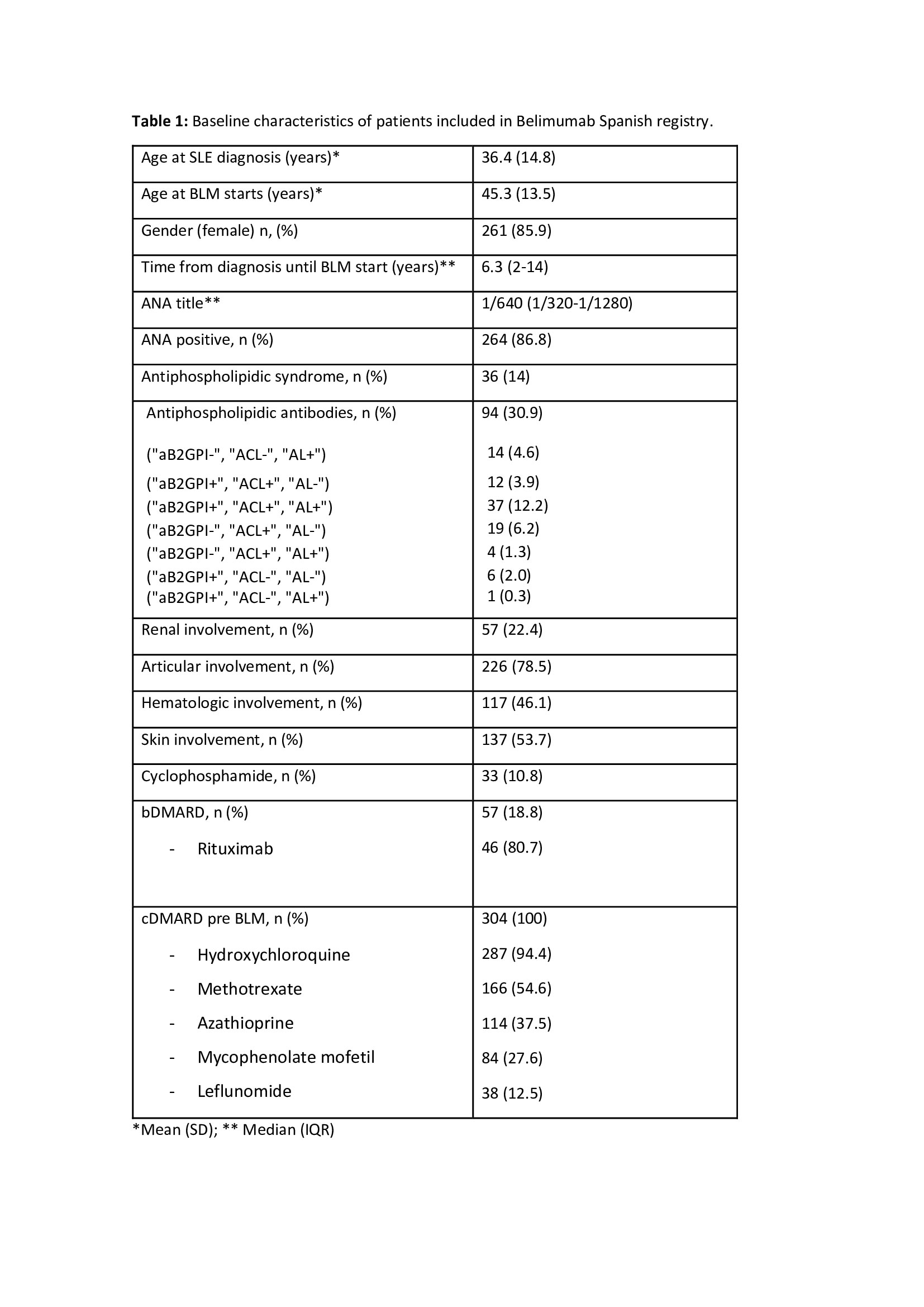
Results: Three hundred and four patients were included. Baseline characteristics of patients are summarized in Table 1.
Regarding administration route 37.2% were on intravenous (IV), 46.2% on subcutaneous (SC) and 16.8% switched from IV to SC route.
The median number of pre-BLM csDMARD use was 2.0 (2.0 to 3.0), being hydroxychloroquine (HCQ) the most frequently used (94.4%). 33 patients were treated with IV cyclophosphamide (CFM) with a median of 6 (4-10) IV bolus received.
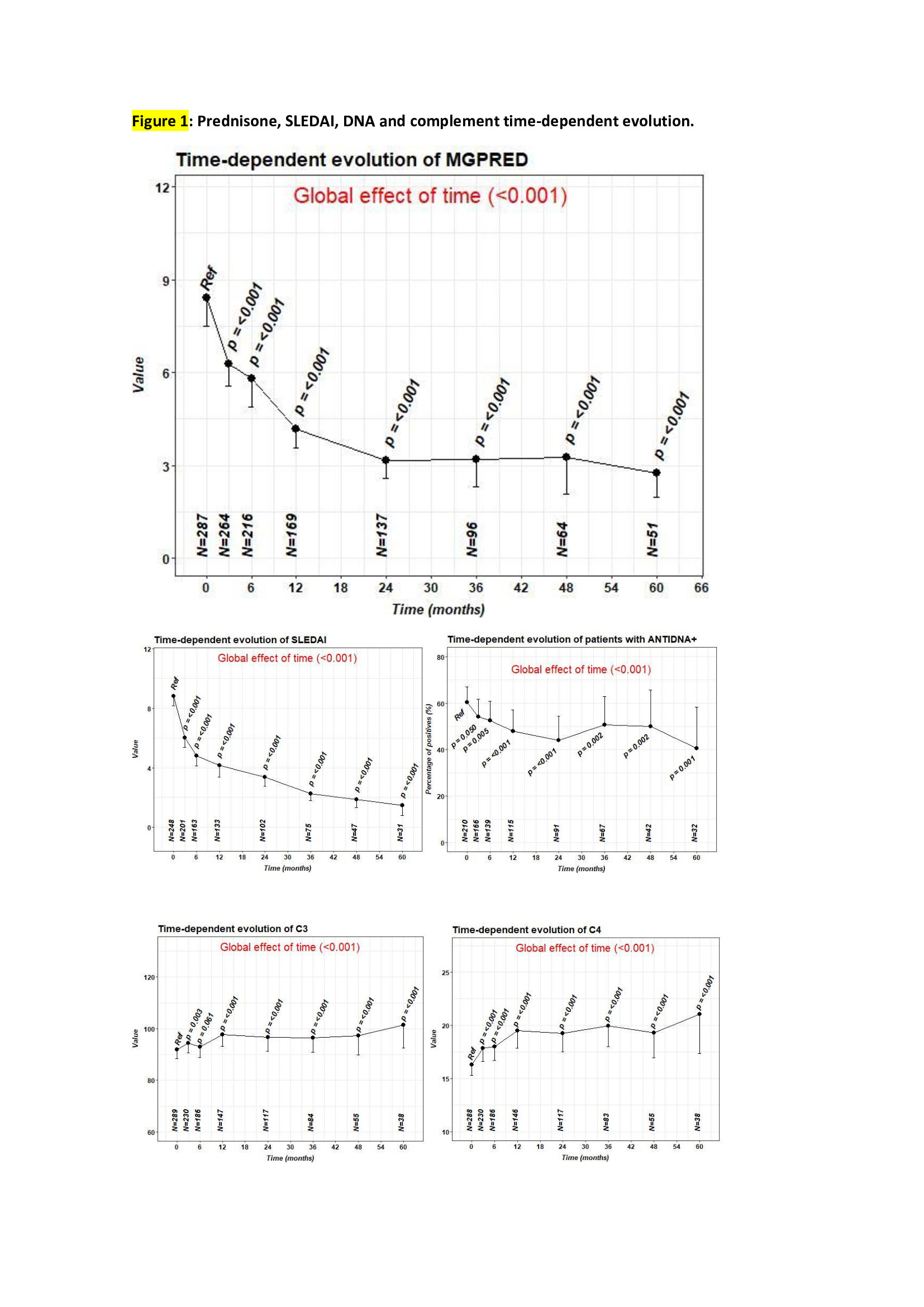
287 patients were on prednisone at the time of BLM start with a median dose of 6.2 (5.0 to 10.0) mg. A statistically significant decrease in prednisone dose, SLEDAI and DNA was observed from baseline until the last visit, while complement C3 and C4 values raised (Fig. 1).
In period1 73 patients started BLM while in period2 started 231. The median time from SLE diagnosis to BLM begin in the period 1 and 2 was 7.0 (3.5-14.5) and 6.3 (1.7-13.9) years, respectively (p=0.164). We found a trend in using less csDMARD before BLM starts between both periods: 3 (2-3) vs. 2 (2-3) (p=0.059).
Fifty seven patients (22.4%) had renal involvement. The median 24 hours proteinuria was 0.6 (0.0 to 1.4) g/day and renal biopsy was done in 48 out of 57 patients, being class V (25.5%), class II (23.4%) and class III (21.3%) the most reported. 73.6% improved after BLM begins with last 24 hour-proteinuria median of 0.2 (0.1 to 0.8) g/day.
One hundred and one patients discontinued treatment mostly due to inefficacy (56.4%) and infections (12.9%). In fact, 54 patients developed infections, most of them mild, 2 patients died, 7 had COVID and 3 patients developed tumors requiring discontinuation of the drug.
Conclusion: In our cohort of patients with SLE, more patients started BLM in the last 6 years than in the first 6 years after its approval. We also found a trend to initiate BLM with less csDMARD use, but the time since SLE diagnosis until BLM start was similar.
A significant improvement in prednisone dose reduction, SLEDAI, complement and anti-DNA was demonstrated. BLM seems to be a good choice for patients with lupus nephritis since nearly three quarters of them normalized proteinuria values. No new safety alarms were reported.
To cite this abstract in AMA style:
Aldasoro V, Laiño M, Enguita M, Castañeda S, Loricera J, Lasa-Teja C, Moriano C, Calvo Río V, Casafont-Solé I, Font Urgelles J, Quiroga-Colina P, Hernandez S, Heredia S, Garcia-Aparicio A, Belzunegui Otano J, Fariña A, Navarro Blasco F, Fanlo Mateo P, Gallego A, Blanco Madrigal J, Matías M, Peralta C, Camins-Fàbregas J, Paulino M, Urruticoechea A, Cossio Jimenez P, Medina Malone M, Perez Pampin E, Ortega-Castro R, varas de Dios B, Lamua Riazuelo J, Giner E. Spanish National Registry of Belimumab in Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/spanish-national-registry-of-belimumab-in-patients-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/spanish-national-registry-of-belimumab-in-patients-with-systemic-lupus-erythematosus/