Session Information
Date: Monday, November 6, 2017
Title: Systemic Lupus Erythematosus – Human Etiology and Pathogenesis Poster I
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Programmed cell death protein 1 (PD-1/CD279) is a cell surface receptor that belongs to the extended CD28/CTLA-4 family and is expressed on T cells and pro-B cells. PD-1 plays an important role in down regulating the immune system by preventing the activation of T-cells. Soluble PD-1 (sPD-1), which is produced by the alternative splicing, can functionally block the regulatory effect of membrane-bound PD-1 on T cell activation. We aimed to retrospectively evaluate the usefulness of serum sPD-1 as a biomarker for systemic lupus erythematosus (SLE).
Methods: In the period from 2010 through 2016, serum samples from 74 hospitalized patients due to active SLE were retrieved from the Tokyo Women’s Medical University SLE Biorepository. Serum samples from 20 patients with systemic sclerosis (SSc) were similarly retrieved from the Tokyo Women’s Medical University SSc Biorepository and served as controls. Sera from 21 healthy subjects were also used as controls. These samples had been previously collected and stored for unspecified medical studies under general consents. All of the SLE and SSc patients fullfiled the 1997 revised ACR criteria for SLE and the 2013 ACR/EULAR criteria for SSc, respectively. We measured the levels of sPD-1 by enzyme-linked immunosorbent assay (ELISA) kit in sera of patients with SLE and SSc, and healthy controls, and compared them. Clinical and laboratory data including the SLE Disease Activity Index 2000 (SLEDAI-2K) scores were also retrospectively collected from the medical records. Associations between the levels of serum sPD-1 and clinical information were retrospectively and statistically analyzed.
Results: In the study population with SLE patients, 69 were female, the median age was 35, the median anti-dsDNA antibodies titer was 9.9, and the median SLEDAI-2K score was 12. The levels of serum sPD-1 in SLE patients with SLEDAI-2K ≥6 were significantly higher than those in SLE patients with SLEDAI-2K <6, patients with SSc, and healthy controls (p < 0.05 in all comparisons), whereas there was no significant difference in other comparisons (Figure). Among the SLE patients, the levels of sPD-1 were moderately correlated with the titers of anti-dsDNA antibodies and the SLEDAI-2K scores, and were moderately and inversely correlated with the levels of C3 and C4. The levels of sPD-1 were significantly higher in SLE patients with arthritis, myositis, rash, mucosal ulcers, fever, thrombocytopenia, or leucopenia than those without (p < 0.05 in all comparisons). In addition, the levels of sPD-1 were higher in patients positive for anti-dsDNA antibodies than those negative among SLE patients with SLEDAI-2K ≥6 (p < 0.01). The sPD-1 levels decreased significantly following treatment among SLE patients (p < 0.05).
Conclusion: The findings from the present study suggested that serum sPD-1 can serve as a new biomarker reflecting disease activity in patients with SLE.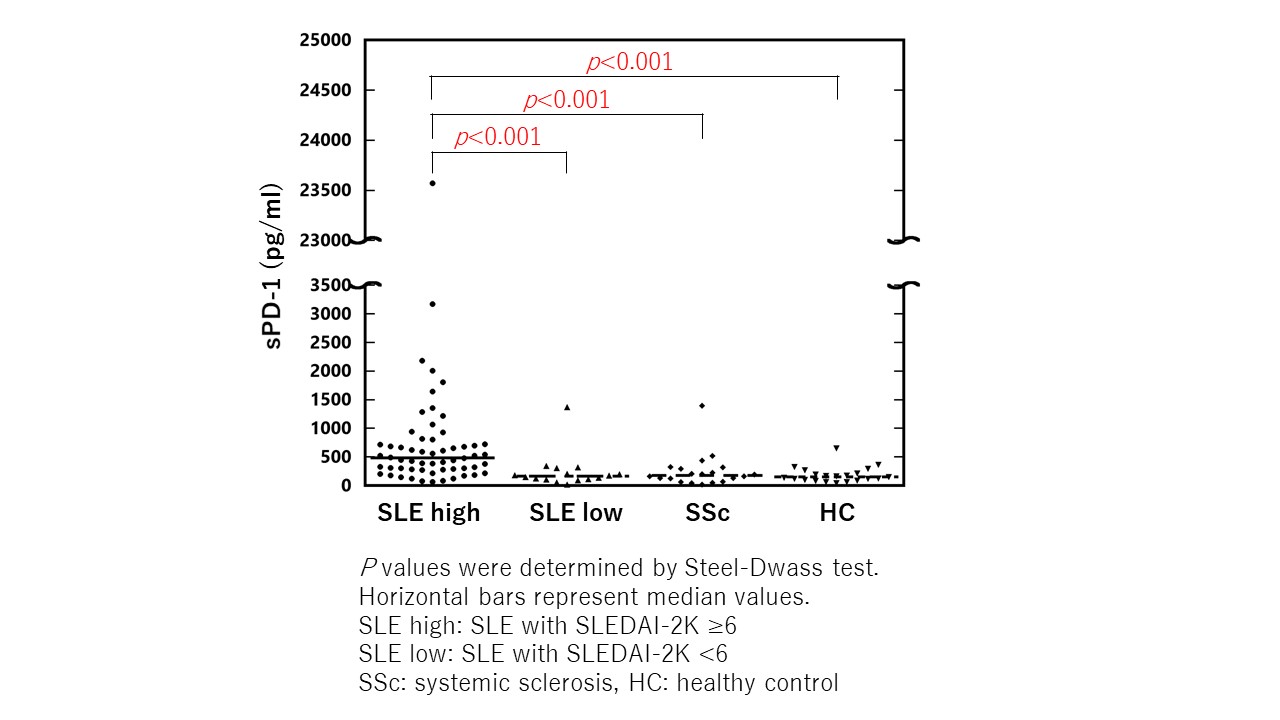
To cite this abstract in AMA style:
Hirahara S, Katsumata Y, Kawaguchi Y, Yamanaka H. Soluble Programmed Cell Death Protein 1 As a Biomarker for Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/soluble-programmed-cell-death-protein-1-as-a-biomarker-for-systemic-lupus-erythematosus/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/soluble-programmed-cell-death-protein-1-as-a-biomarker-for-systemic-lupus-erythematosus/
