Session Information
Date: Sunday, November 13, 2016
Title: Metabolic and Crystal Arthropathies - Poster I: Clinical Practice
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Allopurinol is the most used urate-lowering agent for patients with gout, but around 10% of patients show intolerance to this drug, often at skin, which can be severe. Febuxostat (FBX) has been proposed for allopurinol-intolerant patients due to its different structure. However, data regarding safety in those with previous skin reactions to allopurinol is still limited (Chohan.2011;38:1957. Bardin. 2016;83:314) as these patients were excluded from pivotal trials. The aim was to assess the cutaneous safety of FBX when used in patients with previous skin reactions to allopurinol.
Methods: Retrospective review of patients with crystal-proven gout treated with FBX in our Unit until December 2015. Those with previous skin reaction to allopurinol were selected. We registered epidemiological (age, gender), clinical (skin events), laboratory variables (serum uric acid, glomerular filtration rate), and HLA-B*5801 status. The primary study variable was the rate of patients also presenting skin reactions with FBX. A descriptive analysis with estimation of the 95% confidence interval (95%CI) is presented.
Results: Out 102 gout patients treated with FBX in our Unit, we identified 24 patients with prior allopurinol-related skin events. The median age was 68.5 years (p25-p75 53.7-71.0), being 18 males (75%). Most used starting dose of FBX was 80mg/d (n=16), others were 5mg/d (n=1), 40mg/d (n=4) or 120mg/d (n=3). Median glomerular filtration rate at that time was 77.2 mL/min (62.7-68.6). We identified five patients (20.8%; 95%CI 3-38%) who also developed skin reactions with FBX (see table): in four cases a nonspecific rash, but one suffered from a Stevens-Johnson syndrome. They were HLA-B*5801 negative, and none of these patients presented skin reactions to benzbromarone.
Conclusion: In our series, one out five patients with previous skin reaction to allopurinol also developed after FBX. Larger studies are needed to confirm these results, but this finding strengths caution when using FBX in this subgroup of patients. 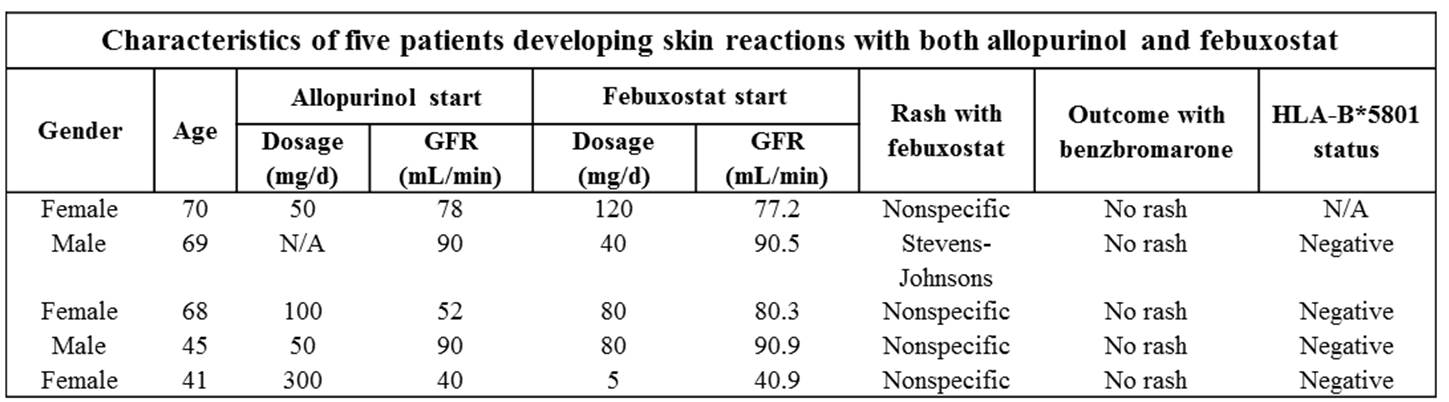
To cite this abstract in AMA style:
Quilis N, Andrés M, Muñoz C, Vela P, Pascual E. Skin Events with Febuxostat in Gout Patients and Previous Skin Reactions to Allopurinol. a Retrospective Review [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/skin-events-with-febuxostat-in-gout-patients-and-previous-skin-reactions-to-allopurinol-a-retrospective-review/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/skin-events-with-febuxostat-in-gout-patients-and-previous-skin-reactions-to-allopurinol-a-retrospective-review/
