Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The sustainability of any regimen
is an important factor to consider when selecting therapy for chronic
conditions, such as rheumatoid arthritis (RA). Recent reports suggest that
patients (pts) treated with abatacept (ABA) might have better retention rates
than those treated with anti-TNFs. We aim to further assess long term retention
rates of ABA in comparison with anti-TNFs in the first and second lines of
treatment in a real life setting using the Rhumadata database and clinical registry.
Methods: RA patients treated at the Institut de recherche
en Rhumatologie de Montréal (IRRM) and the Centre d’Ostéoporose et de
Rhumatologie de Québec (CORQ) with either ABA or an anti-TNF inhibitor,
adalimumab (ADA), etanercept (ETA), or infliximab (INF) as first biologic (first
cohort) or second biologic (second cohort) after January 1st 2007. Descriptive
statistics were used to describe patient characteristics. Characteristics were compared
using ANOVA with Bonferroni correction. Kaplan-Meier methods were used to
compute the cumulative incidence of treatment discontinuation.
Results: The first cohort included 403 pts (62 ABA, 111
ADA, 195 ETA, and 35 INF) and the second cohort included 189 pts (76 ABA, 47
ADA, 47 ETA, and 19 INF). No clinically significant differences in baseline
characteristics were noted between treatment groups. There were no significant
differences in retention rates between ABA and anti-TNFs in the first cohort,
Figure 1. The estimated 6-years drug retention rates were 52.3% (SD=8.4%) for
ABA, 37.8% (SD=4.9%) for ADA, 43.6% (SD=4.3%) for ETA and 45.6% (SD=8.8%) for
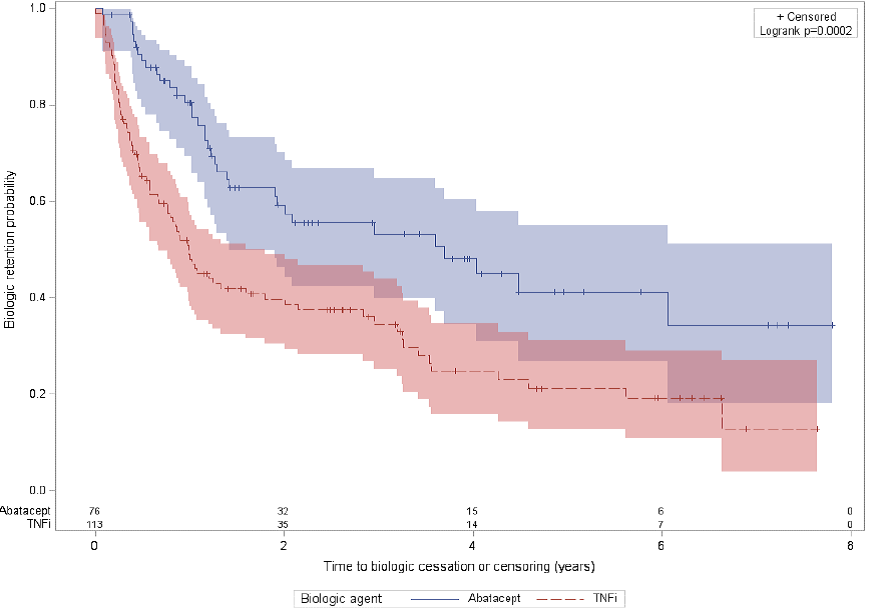
INF. In the second cohort, in patient with RA having failed a first anti-TNF
agent, retention rates with ABA were significantly higher compared to anti-TNFs,
Figure 2. For this cohort, the estimated 6-years drug retention rates were
41.2% (SD=7.4%) for ABA, 15.2% (SD=6.3%) for ADA, 22.7% (SD=7.5%) for ETA and
33.1% (SD=13.1%) for INF. The significantly higher retention rates with ABA in
the second cohort were maintained regardless of RF or anti-CCP status or whether
the biologics were used as monotherapy or in combination with DMARDs. Lack of
efficacy (40.1% and 57.3% in the first and second cohort, respectively) and
adverse effects (13.9% and 12.2% in the first and second cohort, respectively)
were the most commonly cited reasons for discontinuation.
Conclusion: As a first line biologic, in patient with
RA, ABA has similar 6-year retention rates as anti-TNFs. As a second
line biologic, in patient with RA, ABA has significantly higher 6-years
retention rates compared to anti-TNFs.
Figure 1.
Figure 2.
To cite this abstract in AMA style:
Choquette D, Bessette L, Haraoui B, Raynauld JP, Sauvageau D, Turcotte A, Villeneuve , Coupal L. Six-Year Retention Rates with Abatacept Vs TNF Inhibitors in the Treatment of Rheumatoid Arthritis: Experience from the Real-World Rhumadata Clinical Database and Registry [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/six-year-retention-rates-with-abatacept-vs-tnf-inhibitors-in-the-treatment-of-rheumatoid-arthritis-experience-from-the-real-world-rhumadata-clinical-database-and-registry/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/six-year-retention-rates-with-abatacept-vs-tnf-inhibitors-in-the-treatment-of-rheumatoid-arthritis-experience-from-the-real-world-rhumadata-clinical-database-and-registry/