Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Sirukumab (SIR), a human monoclonal antibody that selectively binds to the IL-6 cytokine with high affinity, has demonstrated efficacy in rheumatoid arthritis (RA) using dose regimens of 50mg q4w and 100mg q2w. Maintenance of response throughout the dosing interval is beneficial, especially when treatment is given less frequently. A global Phase 3 study in patients (pts) with active RA refractory to conventional, synthetic, disease-modifying antirheumatic drugs (DMARDs; SIRROUND-D) demonstrated the efficacy and safety of both dose regimens of SIR. Post-hoc analyses of SIRROUND-D were performed to assess response maintenance with SIR 50mg q4w (less frequent dosing) and SIR 100mg q2w (more frequent dosing) over 2w and 4w intervals.
Methods: Pts (n=1,670) were randomized 1:1:1 to SC SIR 50mg q4w, SIR 100mg q2w, or placebo q2w. The primary efficacy endpoint was ACR20 response at Wk 16. Additional efficacy outcomes evaluated included change from baseline (BL) in CDAI, DAS28(CRP), and CRP, evaluated every 2-4w. Serum samples for pharmacokinetics were collected and analyzed at BL up to 52w. Post hoc subgroup analyses of patients who were EULAR Good-Moderate responders at 12w assessed the % of pts with loss of response (improvement ≤1.2 from BL) over 2w and 4w dosing intervals and change from BL in DAS28(CRP) over 2w and 4w intervals by treatment group for study visits up to 52w to determine any change in response based on dosing regimen.
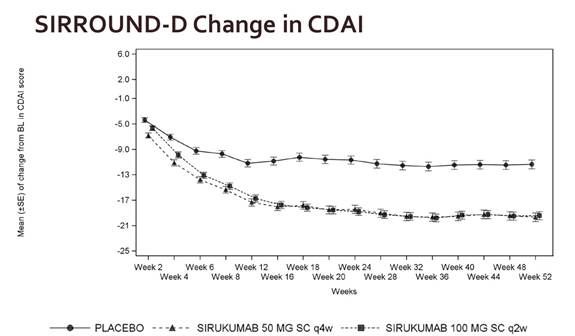
Results: Following multiple SC doses of SIR 50 mg q4w or 100 mg q2w, trough serum SIR concentrations reached steady state (SS) by approximately 12w and remained stable through 52w. ACR20 response and improvements from BL in CRP, CDAI, and DAS28(CRP) were achieved and maintained over 2w intervals from 16w to 20w (post-SS) and through 52w for both SIR dose groups (Fig 1). In pts with EULAR Good-Moderate response at 12w, there was no evidence of a change in mean DAS28(CRP) between Wks 16, 18, and 20 for either the 2w or 4w SIR dose. Furthermore, <3% of pts on SIR 50mg q4w and ≤5% of pts on SIR 100mg q2w lost response at any individual study visit between 16w and 52w. There was no increase in % of pts with loss of response over time, and 80% of pts with loss of response at a study visit had loss of response only at that visit or 1 other visit through 52w. The loss of response rates were similar for SIR 50mg q4w and SIR 100mg q2w.
Conclusion: In a SIR phase 3 clinical trial in RA pts refractory to DMARDs, trough serum SIR concentrations were stable during the time interval that efficacy was assessed in the majority of pts. The q4w and q2w dosing regimens were similar with respect to maintenance of response throughout the different dosing intervals, and the response was effectively maintained to 52w. These data demonstrate that sirukumab q4w dosing provides an effective treatment option for pts with RA.
Fig 1
To cite this abstract in AMA style:
Brooks DM, Plotnick M, Williams NJ, Kurrasch R, Zhuang Y, Tompson DJ, Hsu B, Rao R. Sirukumab Subcutaneous Dose Regimens Maintain Clinical Response over Dosing Intervals in Rheumatoid Arthritis Patients: Results from a Phase 3 Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/sirukumab-subcutaneous-dose-regimens-maintain-clinical-response-over-dosing-intervals-in-rheumatoid-arthritis-patients-results-from-a-phase-3-study/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sirukumab-subcutaneous-dose-regimens-maintain-clinical-response-over-dosing-intervals-in-rheumatoid-arthritis-patients-results-from-a-phase-3-study/