Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Beyond thrombotic events, antiphospholipid syndrome (APS) is also associated with an organ-threatening small-vessel vasculopathy that lacks specific treatments. A soon-to-be-published skin biopsy study focusing on 3 individuals with marked livedo racemosa identified the endothelial cell Hippo-YAP1-CCN2 axis as a key player in APS vasculopathy. This signature was also confirmed in APS kidney biopsies, suggesting that easily accessible dermal tissue at least partially echoes the small-vessel vasculopathy of other organs. Here, we expanded the skin biopsy concept to a larger APS cohort (n=30) with varying clinical manifestations, aiming to discover cell populations and molecular pathways that may herald specific APS endotypes.
Methods: Single-cell RNA sequencing was performed on upper-thigh skin biopsies (epidermis removed) from 30 primary APS patients and 14 healthy controls (Fig 1). Cell type-specific differentially expressed genes (DEGs) were identified by likelihood-ratio tests and pathway enrichment analyses were carried out in g:Profiler. Genes associated with APS clinical manifestations were tested by linear regression and logistic regression (continuous or binary traits, respectively).
Results: Thirteen unique cell types were detected in the dermis, with an increased proportion of endothelial cells in APS patients compared to healthy controls (18% vs. 16%, p< 0.001). Within the endothelial cell population, 6 subpopulations were identified. One subpopulation of capillary endothelial cells (Cap1) was significantly expanded in APS patients (Fig 2). Cap1 demonstrated higher expression of genes like CCL2, CCN2, FN1, and TAGLN, with pathway analysis emphasizing enhanced leukocyte adhesion and cell proliferation. We next asked whether the expression of specific genes in Cap1 was associated with unique clinical phenotypes. As compared with other APS patients, patients with a history of small-vessel events demonstrated upregulation of CD74, B2M, and several MHC class II genes, which together suggest increased immunological communication between leukocytes and endothelial cells. Evidence of increased interferon signaling was also appreciated in the small-vessel patients (Fig 3). When we characterized serum creatinine and urine protein as continuous variables, we found kidney dysfunction to be associated with molecular pathways including hypoxia (SOD2, EDN1) and angiogenesis (TMEM100, ANGPTL4).
Conclusion: Through expanded characterization of dermal endothelial cells spanning 30 APS patients, 14 healthy controls, and a total of 30,775 endothelial cells, we identified a capillary subpopulation associated with leukocyte adhesion and cell proliferation that was significantly expanded in patients relative to controls. Within this subpopulation, cell-cell communication and interferon signaling correlated with a history of small-vessel events. Meanwhile, hypoxia and angiogenesis tracked with clinical signs of nephropathy. Taken together, these data bring us closer to the molecular understanding of APS endotypes that will be necessary to eventually bring precision therapies to our APS clinics.
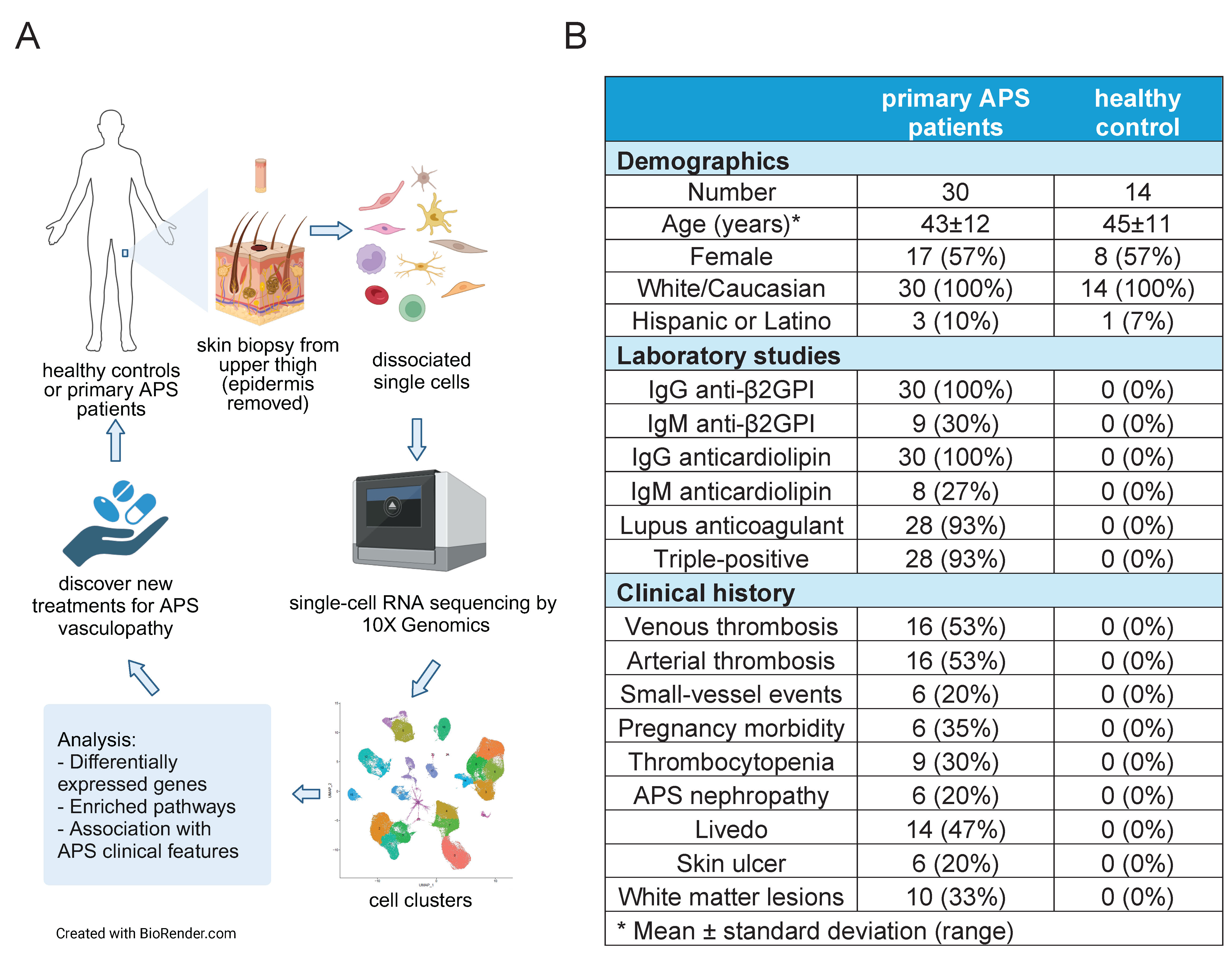
A. Schematic illustration of the study design. Skin samples (epidermis removed) were
collected by upper-leg punch biopsies of 30 primary APS patients and 14 healthy controls.
Single-cell RNA sequencing was performed using 10x Genomics, followed by analysis of
differentially expressed genes, enriched pathways, and clinical associations.
B. Clinical information of 44 skin biopsy donors.
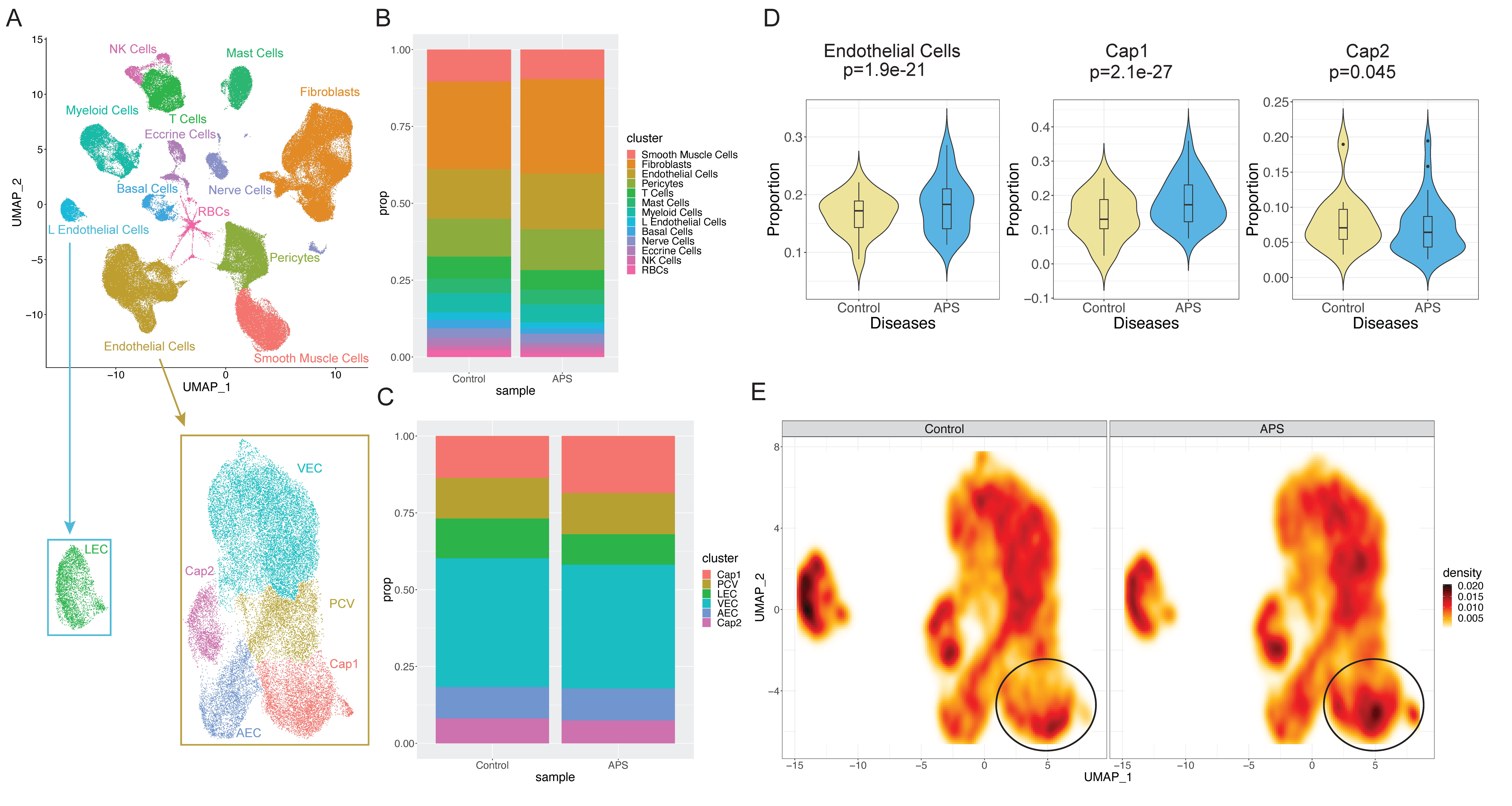
A. Visualization of 13 distinct cell clusters by uniform manifold approximation and projection (UMAP) plot. Sequenced data was analyzed with standard pipelines, including ambient
RNA removal (by SoupX), doublet removal (by scDblFinder), and quality control (by 1.5 interquartile range rule of total number of genes and mitochondrial genes). Harmony
was used for batch-effect correction. Cell annotation was performed based on the established marker genes.
B-C. The cell composition of all cells or endothelial cell subpopulations.
D. Violin plot of cell proportions. Compared to the healthy controls, APS patients had a higher endothelial cell proportion (18% vs. 16%, p<0.001), higher Cap1 proportion (19%
vs. 14%, p<0.001) and lower Cap2 proportion (8% vs. 7%, p=0.045). Cell-type proportion differences between APS and control samples were tested by z-test.
E. Density plot showing the UMAP projection of total endothelial cells in healthy controls and APS patients. Circled areas correspond to Cap1 endothelial subpopulation, where
APS patients manifest a larger proportion compared to controls.
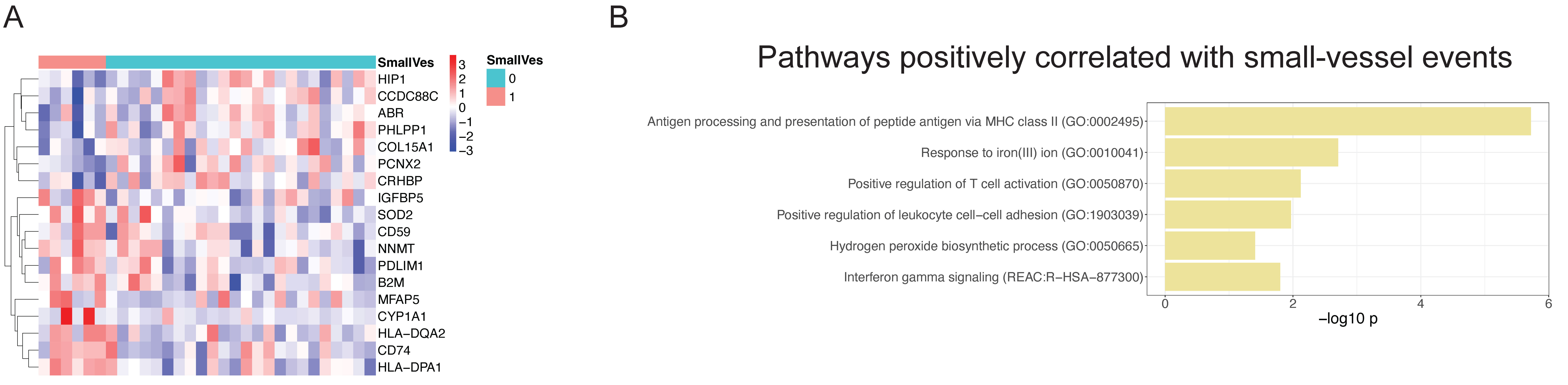
A. Heatmap indicating the statistically significant genes correlated with small-vessel events. The first 6 columns with red color on top indicate 6 patients
with at least one small-vessel event. The last 24 columns with blue-green color on top represent the other 24 patients. The first 7 rows were
genes negatively correlated with small-vessel events, and the last 11 rows were positively correlated genes. Genes associated with binary clinical
traits were tested by logistic regression. Pr(>|t|) <0.05.
B. Selected Gene Ontology (GO) and Reactome pathways positively correlated with small-vessel events.
To cite this abstract in AMA style:
Liang W, Li Q, Madison J, Wasikowski R, Yalavarthi S, Sarosh C, Zuo Y, Tsou P, Tsoi L, Knight J. Single-cell Profiling of Dermal Endothelial Cells Identifies Unique Molecular Pathways Associated with Small-Vessel Events and Nephropathy in Antiphospholipid Syndrome [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/single-cell-profiling-of-dermal-endothelial-cells-identifies-unique-molecular-pathways-associated-with-small-vessel-events-and-nephropathy-in-antiphospholipid-syndrome/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/single-cell-profiling-of-dermal-endothelial-cells-identifies-unique-molecular-pathways-associated-with-small-vessel-events-and-nephropathy-in-antiphospholipid-syndrome/