Session Information
Date: Monday, October 22, 2018
Title: Rheumatoid Arthritis – Treatments Poster II: PROs, Safety and Comorbidity
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Rheumatoid arthritis (RA) is a chronic, systemic inflammatory disorder that causes joint pain and swelling, bone erosions, and deformity. This debilitating disease can severely impact patients’ quality of life, employment opportunities, daily activities, and life expectancy. Over time, RA can cause the joints to become permanently deformed, which may lead to disability. Because of advances in treatment options such as biologic disease-modifying antirheumatic drugs (bDMARDs), many patients with RA are now able to continue to work and lead full lives, whereas decades ago this was not possible. The goal of this study was to assess the impact of introducing bDMARDs as a treatment option for patients with RA.
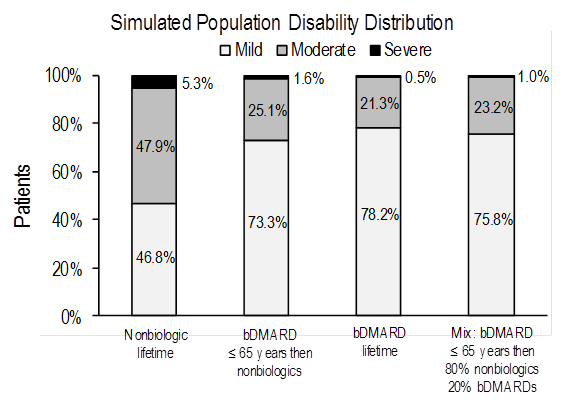
Methods: A population simulation model was developed to assess the impact of different treatment pathways on disability based on clinical trial data and real-world evidence. The model included patients diagnosed with RA with symptoms <2 years (median 6 months) who were DMARD-naïve. The following treatment pathways were evaluated: 1) nonbiologics (including nonsteroidal anti-inflammatory drugs, glucocorticoids, and synthetic DMARDs) for remaining lifetime, 2) bDMARDs for remaining lifetime, 3) bDMARDs until age 65 years then either 100% or 80% of patients switch to nonbiologics while the remaining patients continue on bDMARDs. A cohort of 1,000 patients newly diagnosed with RA, aged 56 years, with mean HAQ score of 1.2 were added every year. We assumed a decrease in mean HAQ of ~0.2 for nonbiologics and ~0.6 for biologics for the first year. For subsequent years, we assumed an annual change in HAQ of +0.002 at ages <65 years and +0.027 at ages ≥65 years for nonbiologics and of ‑0.009 at ages <65 years and +0.016 at ages ≥65 years for bDMARDs. The primary model outcomes were: expected population distribution of disability defined by HAQ score (score <0.5 to <1.1 = mild; score 1.1 to < 2.1 = moderate; score 2.1 to 3.0 = severe disability), and patient life expectancy. The impact of introducing bDMARDs on societal cost will be evaluated in the final analysis.
Results: For each simulated treatment pathway, the figure presents an expected distribution of disability in an RA population at steady state. Individual cohort life expectancy ranged from 22.11 years (nonbiologics) to 24.11 years (bDMARDs). Results were sensitive to starting age and disease severity. Greater benefits (ie, greater reduction in severe disability) associated with bDMARDs compared to nonbiologics can be seen in patients with higher mean HAQ scores entering the simulation model.
Conclusion: The simulation model estimates show that biologics will reduce the RA population disability burden and increase the life span of RA patients. The model can be used to estimate additional population outcomes including costs and healthcare resource use.
To cite this abstract in AMA style:
Mauskopf J, Gharaibeh M, Wamble D, Collier DH, Stolshek BS, Matteson EL. Simulating Population Disability Outcomes for Alternative Treatment Pathways in Patients with Active Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/simulating-population-disability-outcomes-for-alternative-treatment-pathways-in-patients-with-active-rheumatoid-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/simulating-population-disability-outcomes-for-alternative-treatment-pathways-in-patients-with-active-rheumatoid-arthritis/