Session Information
Date: Monday, November 11, 2019
Title: 4M119: Sjögrenʼs Syndrome – Basic & Clinical Science (1902–1907)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
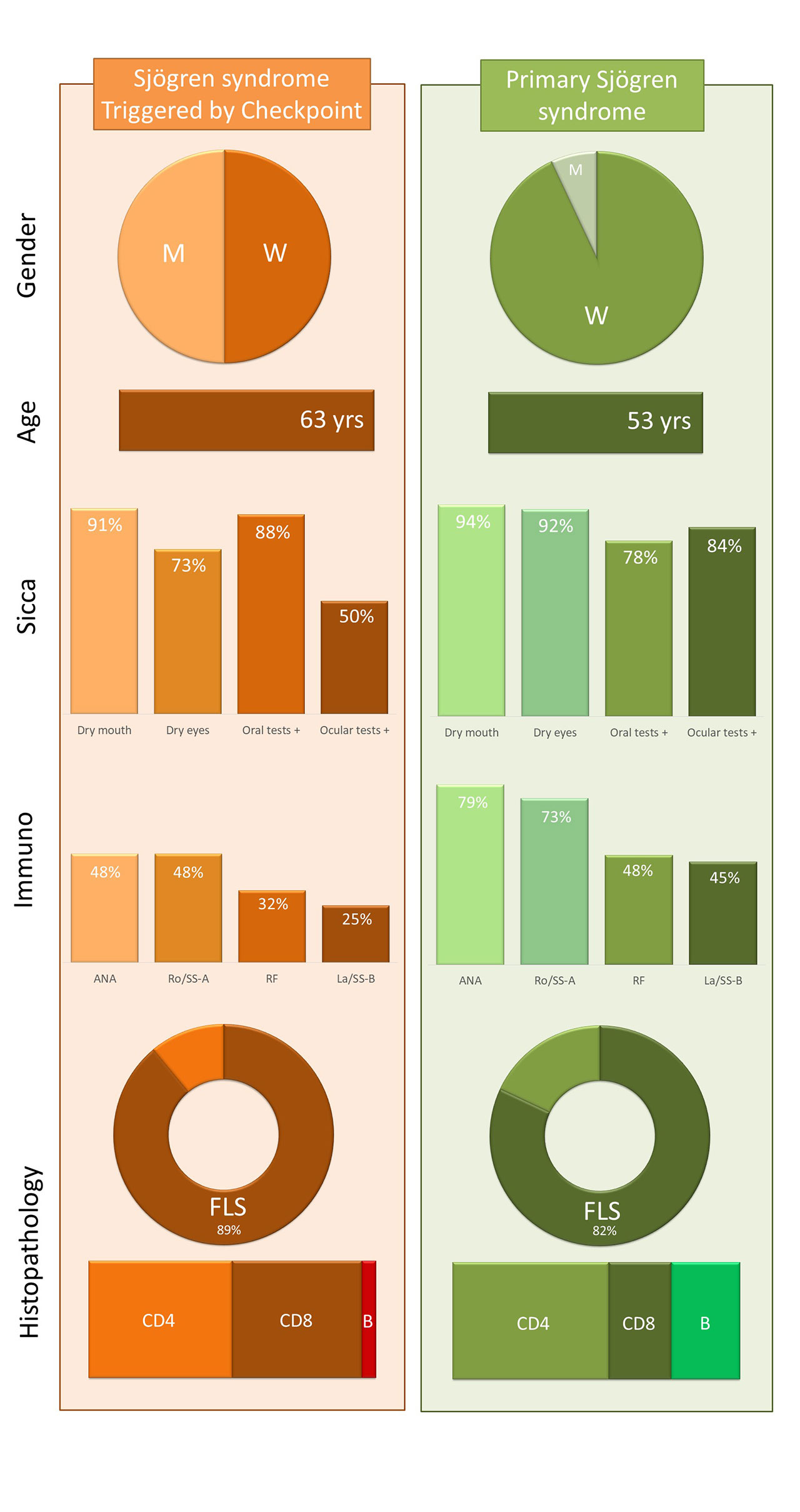
Background/Purpose: To analyse the worldwide occurrence of sicca/Sjögren (SjS) syndrome associated with the use of immune checkpoint inhibitors (ICI) in patients with cancer.
Methods: The ImmunoCancer International Registry (ICIR) is a Big Data-Sharing multidisciplinary network composed by 40 specialists in Rheumatology, Internal Medicine, Immunology and Oncology from 18 countries focused on the clinical and basic research of the immune-related adverse events (irAEs) related to cancer immunotherapies. For this study, patients investigating for a clinical suspicion of SjS after being exposed to ICI were included.
Results: We identified 26 patients (11 women and 15 men, with a mean age at diagnosis of 63,57 years). Underlying cancer included lung (n=12), renal (n=7), melanoma (n=4), and other (n=3) neoplasia. Cancer immunotherapies consisted of monotherapy (77%) and combined regimens (23%). In those patients receiving monotherapy, all patients were treated with PD1/PD1-L inhibitors (nivolumab in 9, pembrolizumab in 7 and durvalumab in 4); no cases associated with CTLA-4 inhibitors were identified. The main SjS-related features consisted of dry mouth in 25 (96%) patients, dry eye in 17 (65%), abnormal ocular tests in 10/16 (62%) and abnormal oral diagnostic tests in 12/14 (86%) patients. Minor salivary gland biopsy was carried in 15 patients: histopathological findings consisted of mild chronic sialadenitis in 8 (53%) patients and focal lymphocytic sialadenitis in the remaining 7 (47%); a focus score was measured in 5 of the 6 patients (mean of 1.8, range 1 to 4). Immunological markers included positive ANA in 13/25 (52%), anti-Ro/SS-A in 5/25 (20%), RF in 2/22 (9%), anti-La/SS-B in 2/25 (8%), low C3/C4 levels in 1/17 (6%) and positive cryoglobulins in 1/10 (10%). Classification criteria for SjS were fulfilled by 10 (62%) out of 16 patients in whom the two key classificatory features were carried out. Among the 26 patients, there were only 3 (11%) who presented exclusively sicca syndrome without organ-specific autoimmune manifestations. Therapeutic management included measures directed to treat sicca symptoms and therapies against autoimmune-mediated manifestations (glucocorticoids in 42%, second/third-line therapies in 31%); therapeutic response for systemic features was observed in 8/11 (73%). No patient died due to autoimmune involvement.
Conclusion: Patients with Sjögren syndrome triggered by ICI display a very-specific profile different from that reported in idiopathic primary SjS, including more frequent occurrence in men, a higher mean age, a predominant immunonegative serological profile, and a notable development of organ-specific autoimmune involvement in spite of the poor immunological profile (Figure). The close association found between sicca/Sjögren syndrome and primarily PD1 blockade requires further specific investigation.
To cite this abstract in AMA style:
Ramos-Casals M, Maria A, Suárez-Almazor M, Lambotte O, Fisher B, Hernandez-Molina G, Guilpain P, Pundole X, Retamozo S, Flores-Chávez A, Baldini C, Bingham C, Brito-Zerón P, Gottenberg J, Kostine M, Radstake T, Schaeverbeke T, Schulze-Koops H, Calabrese L, Khamashta M, Mariette X. Sicca/Sjögren Syndrome Triggered by PD-1/PD-L1 Checkpoint Inhibitors: Data from the International ImmunoCancer Registry (ICIR) [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/sicca-sjogren-syndrome-triggered-by-pd-1-pd-l1-checkpoint-inhibitors-data-from-the-international-immunocancer-registry-icir/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sicca-sjogren-syndrome-triggered-by-pd-1-pd-l1-checkpoint-inhibitors-data-from-the-international-immunocancer-registry-icir/