Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
Anti-TNFs have greatly contributed to improve RA prognosis. Hence, the needs for orthopedic surgery have considerably decreased in the past years. However, surgery, whether programmed or not, whether orthopedic or not, is sometimes necessary in patients treated with TNF inhibitors. Current recommendations are to discontinue biologic DMARDs to reduce the risk of surgical site infection. The guidelines for periopetrative cessation periods of biologic DMARDs differ among countries but generally a stop of the biotherapy 2 to 6 weeks before programmed surgery is recommended. Anti-TNF can be resumed after wound healing.
The purpose of this current study was to ask:
- Whether patients treated with anti-TNF are really at risk for infection upon surgery.
- Whether stopping anti-TNF treatment increases the risk of infection upon surgery.
Methods
We have conducted a systematic review of the literature indexed in Pubmed, Embase, and Cochrane using the following keywords: “Rheumatoid arthritis AND surgery AND infection AND (adalimumab OR certolizumab OR etanercept OR golimumab OR infliximab)”. This search was conducted up to February 2014 and was limited to papers in English and French languages. Reviews and case reports were excluded.
We selected studies reporting numbers of infections observed post-surgery by
i) comparing patients using anti-TNF with patients using DMARDS without biologicals and
ii) comparing patients keeping up with anti-TNF with patients who interrupted anti-TNF before surgery.
Results
Fourteen studies reported the frequency of post-surgery infections of RA patients undergoing orthopedic surgery, most often joint replacement.
Eleven studies were pooled in order to evaluate the infection risk in patients treated with anti-TNF compared with patients treated with DMARD without biologics. There were 4,925 surgeries and 121 infections in patients using anti-TNF and 60,678 surgeries and 711 infections in patients with DMARD alone. Thus, patients treated with anti-TNF are at higher risk of post-surgery infection (OR = 1.95 [1.34-2.85]). Some joints such as foot, ankle and elbow seem to be at higher risk.
Six studies were pooled for meta-analysis evaluating the benefits of stopping anti-TNF as post-infection risk is concerned. Two studies were not informative, as no infection was reported in any of them. Stopping the anti-TNF treatment did not modify the infection risk: OR = 0.70 [0.39-1.25].
Conclusion
This meta-analysis shows that patients treated with anti-TNF are more exposed to risks of infection after orthopedic surgery. The increased rate of infection in those patients was not ameliorated by stopping anti-TNF before surgery. This could lead clinicians, at least in surgeries with lower risks of infection, to reconsider stopping anti-TNF before surgery since this may expose patients to a flare-up.
Acknowledgement: We wish to thank AbbVie who provided logistic support.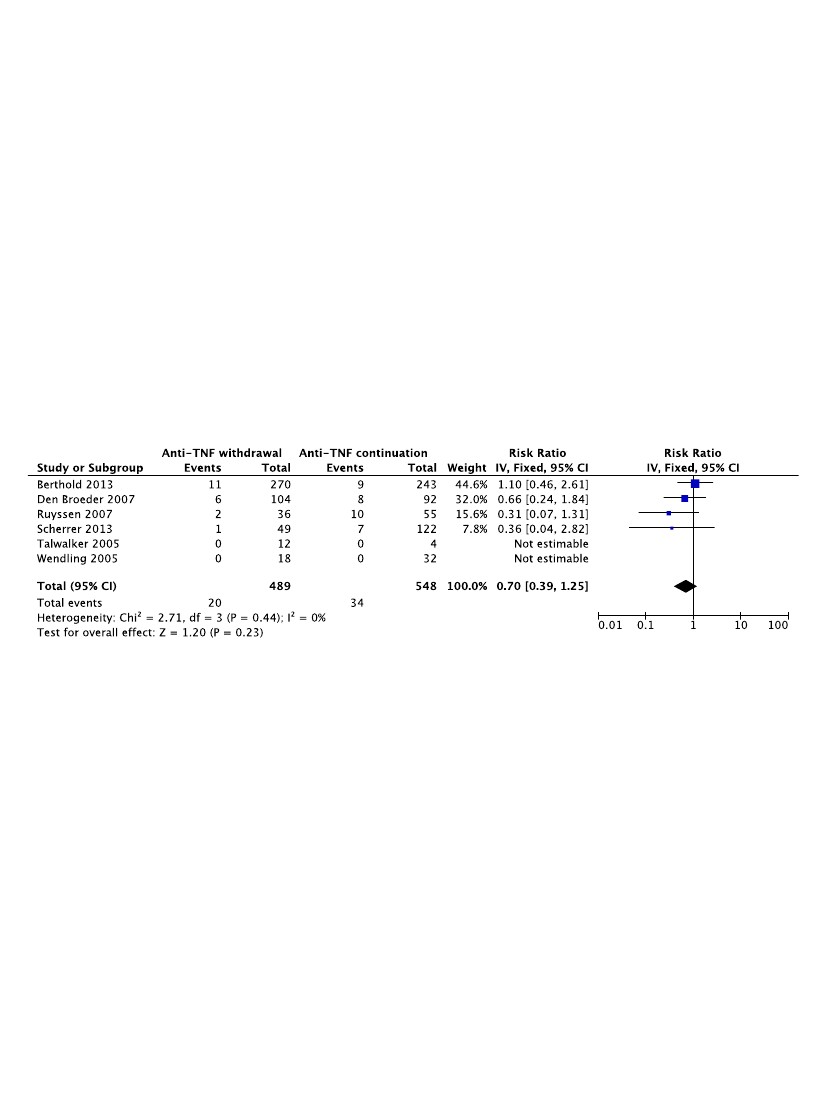
Disclosure:
C. Mabille,
None;
A. Ruyssen Witrand,
None;
T. Barnetche,
None;
A. Constantin,
None;
A. G. Cantagrel,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/should-anti-tnfa-treatment-of-ra-be-stopped-before-orthopedic-surgery/
