Session Information
Date: Sunday, November 5, 2017
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Juvenile idiopathic arthritis (JIA) comprises 7 ILAR categories, but systemic-onset JIA (sJIA) appears to be distinct in genetic background and pathogenesis from the other categories of JIA. The aim of this study is to investigate real-world therapeutic short-term responses in patients with sJIA starting tocilizumab (TCZ) or anakinra (ANK), the 2 most common biologics used currently for this ILAR category.
Methods: This analysis included patients with sJIA enrolled in the UK Biologics for Children with Rheumatic Diseases (BCRD) study starting TCZ or ANK from 01/01/2010 (study start date), with data available at baseline, 6 months and 1 year by 31/12/2016. Disease activity was assessed at baseline, 6 months and 1 year, including outcomes; minimal disease activity (MDA), clinically inactive disease (CID) and ACRPedi90. Univariable logistic regression was used to identify baseline characteristics associated with the outcomes. Multiple imputation was used to account for missing data.
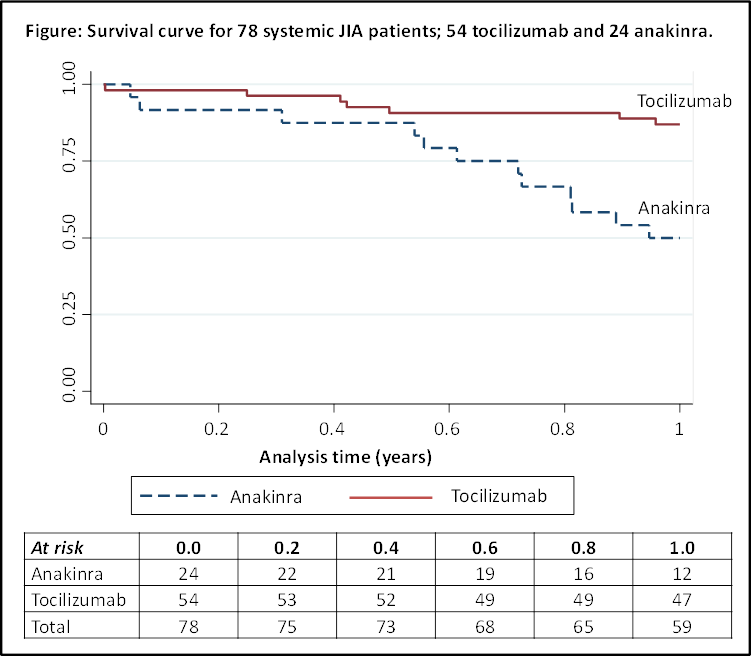
Results: A total of 78 sJIA patients were included (54 TCZ; 24 ANK) (Table); 55% were female and 70% received this drug as their first biologic. Patients starting ANK had a shorter disease duration (0 vs 2 years; p=0.003), and more had a history of macrophage activation syndrome (MAS) (38% vs 8%; p=0.002). Response rates between the 2 drugs were similar. In the whole group, at 1 year, 54% achieved ACRPedi90, 47% MDA and 33% CID (Table). Mean JADAS-71 change was -14 (p<0.001). No baseline characteristics were associated with achieving response. Nineteen (24%) patients stopped their biologic treatment by 1 year (Figure), for reasons including remission (N=1), inefficacy (N=6), adverse events (N=10, including one case of MAS in patient receiving TCZ) and unknown (N=2). Treatment survival was better with TCZ (87% at 1 year vs 50% ANK), with 4 children stopping for injection-related problems.
Conclusion: In this real-world cohort of children with sJIA receiving TCZ or ANK, approximately half the patients achieved a significant clinical short-term response, and one-third achieved inactive disease within 1 year. Although numbers in this analysis are low, reflecting the rarity of sJIA, it is important to report these reassuring real-world outcomes and similarities in response with other JIA categories. As this UK JIA study continues to develop, future analyses investigating longer term tolerability and safety will be possible.
Table: Baseline characteristics and one year outcomes of 78 systemic JIA patients; 54 tocilizumab and 24 anakinra.
|
|
Anakinra (IL-1) N=24 |
Tocilizumab (IL-6) N=54 |
Total N=78 |
p-value |
|
Female, n (%) |
15 (63%) |
28 (52%) |
43 (55%) |
P=0.4 |
|
First Biologic, n (%) |
19 (86%) N=22 |
34 (63%) |
53 (70%) N=76 |
P=0.04 |
|
Age (years), median (IQR) |
6 (2, 13) |
7 (4, 11) |
7 (3, 12) |
P=0.8 |
|
Disease Duration (years), median (IQR) |
0 (0, 1) N=23 |
2 (1, 3) |
1 (0, 2) N=77 |
P=0.003 |
|
Systemic Features Present, n (%) |
13 (81%) N=16 |
24 (53%) N=45 |
37 (61%) N=61 |
P=0.05 |
|
History of Macrophage Activation Syndrome, n (%) |
8 (38%) N=21 |
4 (8%) N=49 |
12 (17%) N=70 |
P=0.002 |
|
Concomitant Oral Steroids, n (%) |
15 (63%) |
36 (67%) |
51 (65%) |
P=0.7 |
|
Concomitant Methotrexate, n (%) |
20 (83%) |
44 (81%) |
64 (82%) |
P=0.8 |
|
Disease Activity, median (IQR) |
|
|
|
|
|
Active Joints (0-71) |
4 (1, 11) N=19 |
4 (1, 8) N=48 |
4 (1, 9) N=67 |
P=0.9 |
|
Physician Global of Disease (0-10cm VAS) |
3 (2, 6) N=16 |
4 (2, 6) N=34 |
3 (2, 6) N=50 |
P=0.8 |
|
Parent Global of Wellbeing (0-10cm VAS) |
4 (1, 5) N=18 |
4 (2, 7) N=34 |
4 (1, 7) N=52 |
P=0.9 |
|
Childhood Health Assessment Questionnaire (0-3) |
1.0 (0.4, 2.0) N=15 |
0.9 (0.4, 1.8) N=34 |
0.9 (0.4, 1.8) N=49 |
P=0.8 |
|
Erythrocyte sedimentation rate, mm/hr |
55 (27, 86) N=19 |
26 (10, 58) N=49 |
35 (11, 67) N=68 |
P=0.1 |
|
JADAS-71 |
18 (6, 29) N=12 |
20 (11, 26) N=22 |
19 (7, 27) N=34 |
P=0.7 |
|
Six Month Outcomes*, % |
|
|
|
|
|
Mean JADAS-71 change |
-10.4 (p=0.1) |
-10.8 (p<0.001) |
-10.7 (p<0.001) |
P=0.9 |
|
ACR Pedi 90 |
51% |
58% |
56% |
P=0.7 |
|
Minimal disease activity (MDA) |
45% |
50% |
49% |
P=0.7 |
|
Clinically inactive disease (CID) |
15% |
20% |
19% |
P=0.7 |
|
Twelve Month Outcomes*, % |
|
|
|
|
|
Mean JADAS-71 change |
-13.3 (p<0.001) |
-13.7 (p<0.001) |
-13.6 (p<0.001) |
P=0.7 |
|
ACR Pedi 90 |
37% |
62% |
54% |
P=0.1 |
|
Minimal disease activity (MDA) |
41% |
49% |
47% |
P=0.5 |
|
Clinically inactive disease (CID) |
22% |
38% |
33% |
P=0.2 |
|
*Using imputed data. Interquartile range (IQR), visual analogue scale (VAS), 71-joint juvenile arthritis disease activity score (JADAS-71), American college of rheumatology paediatric criteria for 90% improvement (ACR Pedi 90). |
||||
To cite this abstract in AMA style:
Kearsley-Fleet L, De Cock D, Baildam E, Beresford MW, Foster HE, Southwood TR, Thomson W, Hyrich KL. Short-Term Outcomes in Patients with Systemic-Onset Juvenile Idiopathic Arthritis Treated with Either Tocilizumab or Anakinra in a Real-World Setting in the United Kingdom [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/short-term-outcomes-in-patients-with-systemic-onset-juvenile-idiopathic-arthritis-treated-with-either-tocilizumab-or-anakinra-in-a-real-world-setting-in-the-united-kingdom/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/short-term-outcomes-in-patients-with-systemic-onset-juvenile-idiopathic-arthritis-treated-with-either-tocilizumab-or-anakinra-in-a-real-world-setting-in-the-united-kingdom/