Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose:
Systemic juvenile idiopathic arthritis (SJIA) is a severe subtype of JIA, which includes systemic features such as fever, rash, elevated inflammatory markers along with poly-arthritis. It is frequently resistant to treatment. Until recently, patients were treated mainly with large doses of glucocorticoids. Breakthroughs in understanding the pathogenesis of SJIA have led to the evaluation of biologics, which block the pro-inflammatory cytokines interleukin (IL)-1 or IL-6 and their receptors.
Our objective was to compare the short-term efficacy of biologics in SJIA [1].
Methods:
A systematic search in MEDLINE, EMBASE, CENTRAL and clinicaltrials.gov was performed. Eligible Randomized controlled trials (RCTs) included patients with SJIA where biologics, at any dose, were compared with another biologic or placebo. In SJIA a modified ACR pediatric (ACRPedi) response is often used with the required addition that systemic features need to be abrogated. Efficacy was evaluated with modified ACRPedi30, 50, and 70. Two reviewers extracted data and a third confirmed data. The network meta-analysis was based on a mixed-effects logistic regression model (modeled in SAS) [2] combining statistical inference from both direct and indirect comparisons of the treatment effects between biologics. Results were reported as odds ratios with 95% confidence intervals (OR [95%CI]).
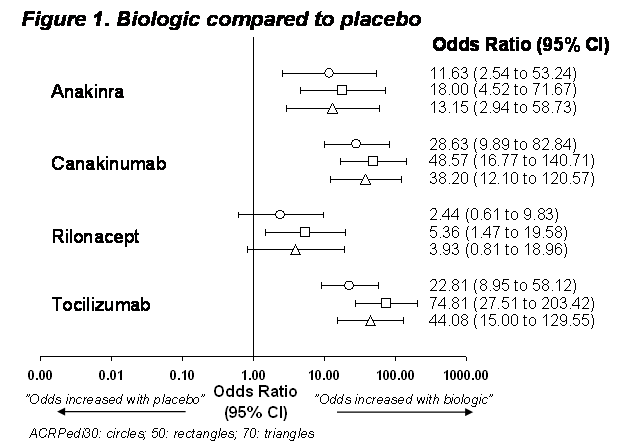
Results:
Of 490 references identified, 23 were reviewed in detail, and 4 RCTs were eligible for inclusion: anakinra, canakinumab, rilonacept and tocilizumab, one trial each all vs. placebo. Rilonacept was statistically significantly (P < 0.05) different from placebo for ACRPedi50, but not for ACRPedi30 or 70 (P > 0.05); anakinra, canakinumab and tocilizumab were statistically superior to placebo for ACRpedi 30,50 and 70 , Figure 1. Comparing biologics, rilonacept treated patients were statistically less likely to respond than those treated with canakinumab or tocilizumab, Figure 2. All other biologics were comparable revealing no statistical signal (P > 0.05).
Conclusion :
This network meta-analysis of short term RCTs presents empirical evidence that canakinumab and tocilizumab were more effective than rilonacept. These results might be influenced by factors such as various study durations (tocilizumab 12 weeks; all other only 4 weeks), and variation in definitions of modified ACRPedi across trials.
References:
[1] Amarilyo G, et al. PROSPERO: International prospective register of systematic reviews. 2013; www.crd.york.ac.uk/PROSPERO/, CRD42013004736
[2] Singh JA, et al. CMAJ. 2009;181(11):787-96
Disclosure:
S. Tarp,
None;
G. Amarilyo,
None;
I. Foeldvari,
Abbott, Chugai,
5,
Pizer,
8;
N. Cohen,
None;
T. D. Pope,
None;
J. M. P. Woo,
None;
R. Christensen,
Abbott, Axellus A/S, Bristol-Myers Squibb, Cambridge Weight Plan, Norpharma, Pfizer, and Roche,
5,
Axellus A/S, Cambridge Weight Plan, Mundipharma, and Roche,
2,
Abbott, Axellus, Bayer HealthCare Pharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Cambridge Weight Plan, Ipsen, Laboratoires Expanscience, MSD, Mundipharma, Norpharma, Pfizer, Roche, and Wyeth,
8;
D. Furst,
AbbVie, Actelion, Amgen, BMS, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech, UCB,
2,
AbbVie, Actelion, Amgen, BMS, Janssen, Gilead, GSK, NIH, Novartis, Pfizer, Roche/Genentech, UCB,
5,
AbbVie, Actelion, UCB ,
8.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/short-term-efficacy-of-biologic-agents-in-patients-with-systemic-juvenile-idiopathic-arthritis-network-meta-analysis-of-randomized-trials/