Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
According to EULAR recommendations, in patients with peripheral arthritis and an inadequate response to at least one conventional synthetic DMARD, biologic DMARDs targeting IL-12/23, IL-17 pathways may be used in patients for whom TNF inhibitors are inappropriate. The objective of this study was to investigate comparative efficacy, safety and tolerability of IL-6, IL-12/23 or IL-17 inhibitors for patients with active psoriatic arthritis (PsA).
Methods:
Randomized controlled trials (RCTs) evaluating the efficacy, safety and tolerability of IL-6, IL-12/23 or IL-17 inhibitors were identified by a comprehensive systematic literature review. Pair-wise meta-analyses and Bayesian network meta-analyses using the random-effects model were performed to estimate pooled odds ratios (ORs) and 95% credible interval (CrI) of attaining a 20% improvement according to American College of Rheumatology criteria (ACR20) and ACR 50 response across trials.
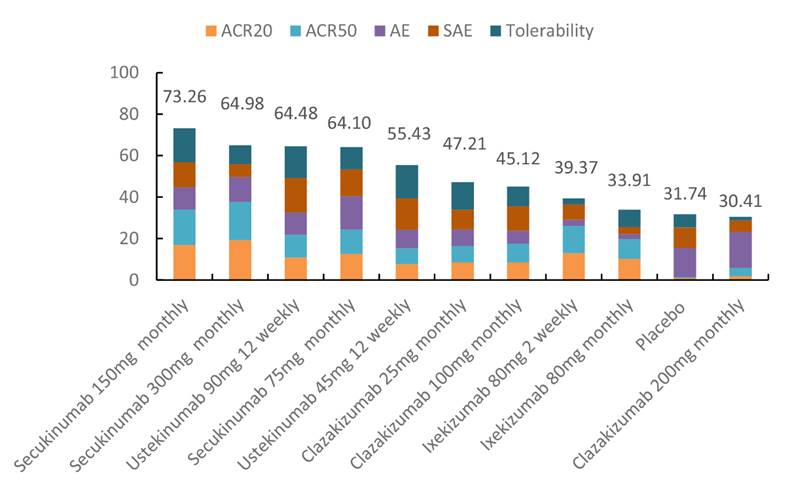
Results:
Six trials were identified which included 2,411 participants and 11 treatments. Pair-wise meta-analysis showed that secukinumab, ustekinumab and ixekizumab demonstrated superior efficacy over placebo in achieving ACR 20 and ACR50 response. However, Ixekizumab has a higher incidence of adverse events (AE) than placebo. In contrast, ustekinumab has a higher tolerability (less likely to be discontinued due to AE) than placebo. Network meta-analysis showed that secukinumab (300mg monthly) had the highest efficacy in achieving ACR20 and ACR50; whereas clazakizumab (200mg monthly), ustekinumab (45mg 12 weekly), secukinumab (150mg monthly) had the lowest probability of having AE, serious AE, and intolerability respectively. Considering overall risk-benefit profile, secukinumab (150mg monthly) may offer an optimal balance for active PsA patients.
Conclusion:
From available evidence, secukinumab was found to be the safest and most efficacious short-term treatments for active PsA amongst all the new biologics targeting the IL-6, IL-12/23 and IL-17 pathways.
Disclosures: This study has partly presented at EULAR2017.
To cite this abstract in AMA style:
Wu D, Yue J, Tam LS. Short-Term Efficacy and Safety of New Biological Agents Targeting the IL-6, IL-12/23 and IL-17 Pathways for Active Psoriatic Arthritis: A Network Meta-Analysis of Randomised Controlled Trials [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/short-term-efficacy-and-safety-of-new-biological-agents-targeting-the-il-6-il-1223-and-il-17-pathways-for-active-psoriatic-arthritis-a-network-meta-analysis-of-randomised-controlled-trials/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/short-term-efficacy-and-safety-of-new-biological-agents-targeting-the-il-6-il-1223-and-il-17-pathways-for-active-psoriatic-arthritis-a-network-meta-analysis-of-randomised-controlled-trials/