Session Information
Date: Monday, November 18, 2024
Title: SLE – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: SLE patients have increased risk of invasive pneumococcal disease due to immune dysregulation and drugs used in these patients. EULAR 2019 recommendation suggest sequential vaccination with the conjugate vaccine followed by PPSV23 for SLE patients. However, the data on immunogenicity of sequential vaccination in SLE is very limited. The sequential vaccine is more expensive and logistically difficult in low- and middle-income countries (LIMC). Thus, we compared the immunogenicity of the two regimens in SLE patients.
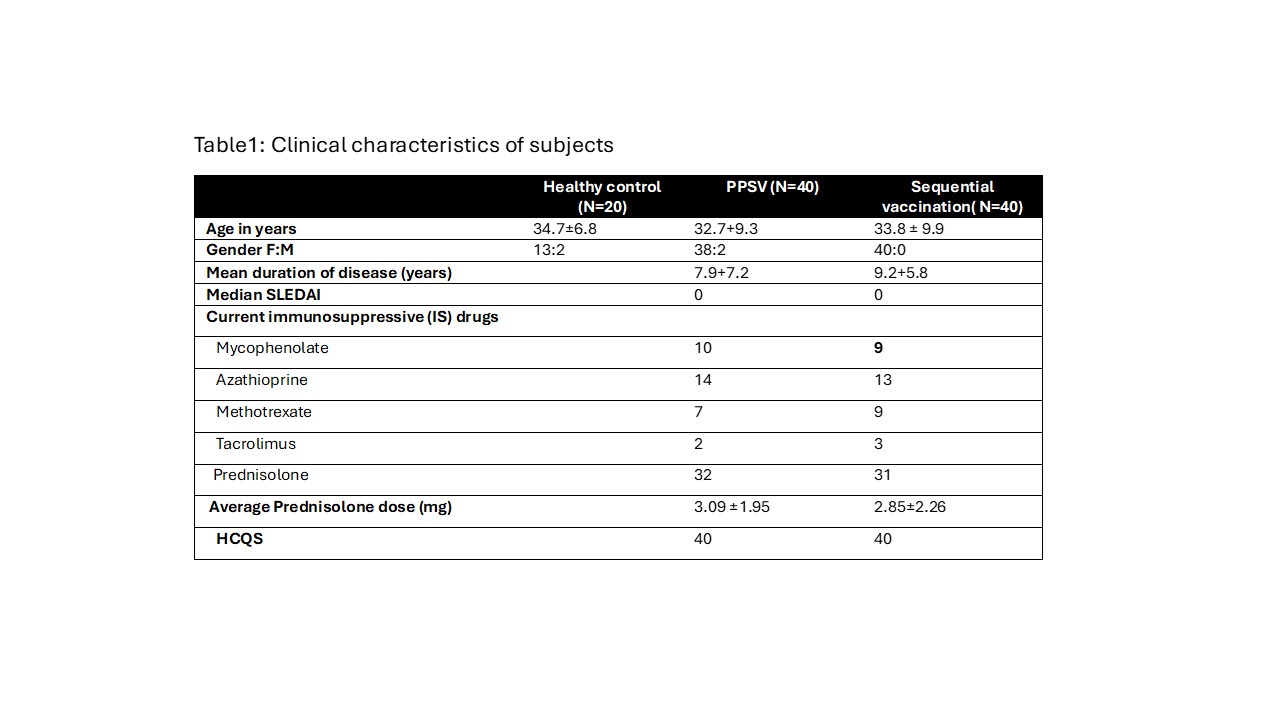
Methods: Adult patients with SLE (ACR/EULAR 2019 criteria) with no change in disease activity (SLEDAI) and background immunosuppressive drugs in last 3 months and on a stable dose of prednisolone ≤10 mg/day were included after informed consent. Participants in sequential arm received 10-valent conjugate pneumococcal vaccine followed by the PPSV23 after 3 months. The second arm received only the PPSV23 vaccine. Baseline and 12-14 weeks post-vaccination samples were used to assess immunogenicity of the vaccine. Antibody responses to 5 serotypes common in India (1, 5, 6B,14 and 19F) of the pneumococci were measured using ELISA. A positive response was defined as at least 2-fold increase from baseline in 3 out of the 5 serotypes tested. Opsonopagocytic assay was done to assess opsonic index of antibodies in some samples. In addition, side-effects and disease flares (SELENA SLEDAI flare index) was also assessed. 40 subjects were enrolled in each vaccine arm. In addition, 15 healthy adult subjects were enrolled for studying the response to PPSV23.
Results: Among 40 in PPSV23 arm 2 were lost to follow and 3 patients baseline serum samples could not be traced in the lab as they were collected during COVID time. Among 40 in sequential arm 37 patients came for next vaccine while 3 patients were lost to follow up. Among 37, one refused 2nd dose, 1 was found to be pregnant, and in 1 patient the dose was delayed due to UTI. The baseline parameters were comparable among the two groups (Table 1)
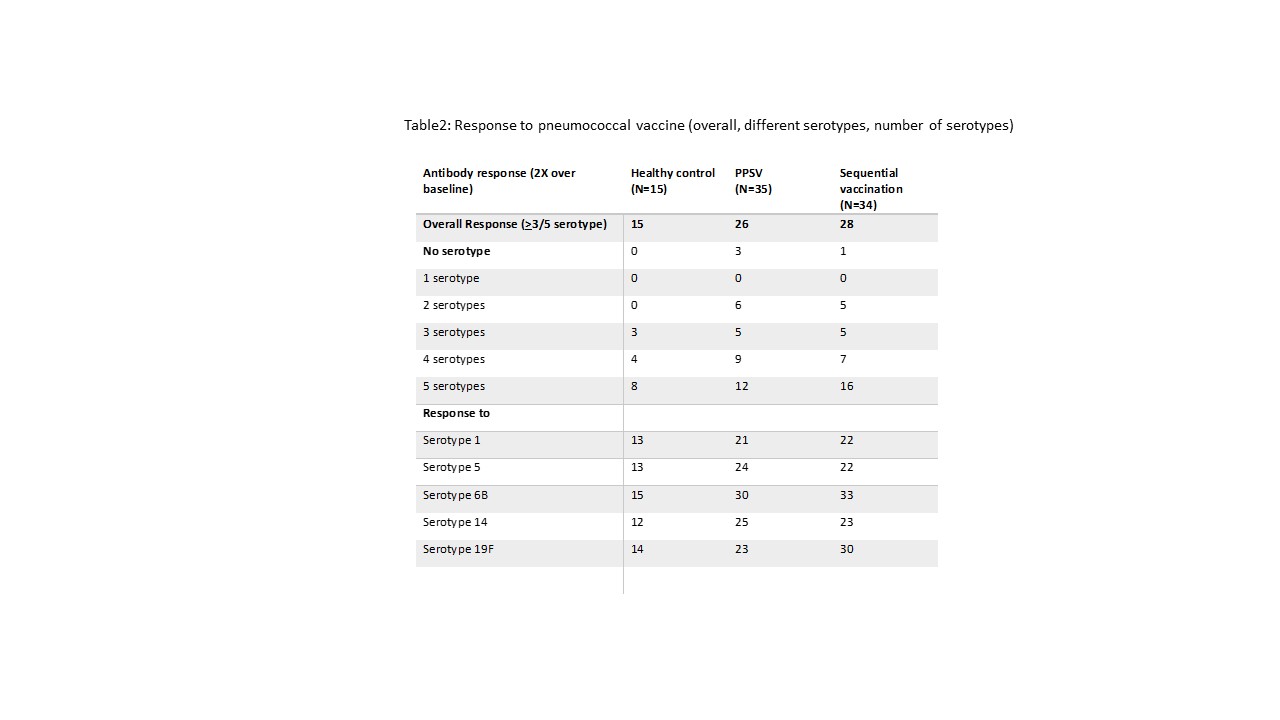
Patients with SLE had a poorer response to PPSV23 as compared to healthy controls (26/35 versus 15/15, p=0.03). Patients vaccinated with sequential vaccine had numerically better response as compared to PPSV23 (28/34 [82.35%] versus 26/35 [74.28%] [Table 2]. Antibodies generated improved opsonic index. The average fold change in all 5 serotypes was not different between sequential and PPSV23 vaccination. All 15 non-responders were on prednisolone while among responders only 41/54 (76%) were on prednisolone but there was no difference in immunosuppressive usage.
In sequential vaccination, 1 patient each had pain at injection site, migraine, fever, fatigue after conjugate vaccine dose and 1 had fever after receiving PPSV 23. 1 patient who had NPSLE in past developed Miller Fisher variant of GBS 4 months after conjugate vaccine dose. In PPSV group 1 each had pain & swelling at injection site, Herpes zoster, headache & fever. None of the healthy control had any side effects.
In PPSV23 arm 3 minor flares were seen while in sequential arm 1 major and 1 minor flare was seen over 6 months.
Conclusion: Both vaccination strategies are safe and give adequate antibody response. Thus, in LMIC a single dose of PPSV23 may be adequate.
To cite this abstract in AMA style:
Chatterjee R, Kommaraju S, MR S, Aggarwal A. Sequential Pneumococcal Vaccination in SLE: Immunogenicity, Side Effects and Comparison with PPSV23 Vaccination [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/sequential-pneumococcal-vaccination-in-sle-immunogenicity-side-effects-and-comparison-with-ppsv23-vaccination/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/sequential-pneumococcal-vaccination-in-sle-immunogenicity-side-effects-and-comparison-with-ppsv23-vaccination/