Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Psoriasis (Pso) and psoriatic arthritis (PsA) are complex, multi-system diseases for which an ever-increasing landscape of therapies exists. Little is known about the population level time-trends in use of these agents. We aimed to examine trends in the use of systemic therapy – both conventional and biologic drugs – for psoriasis and psoriatic arthritis.
Methods: Using claims data (2004-2013) from a large nationwide US commercial health plan, we selected patients with 1) Pso without PsA and 2) PsA based on 2 diagnosis codes separated by 7-365 days. In each group, we identified 3 hierarchical treatment groups based on their topical or systemic treatment in a 12-month baseline period prior to the 2nd PsA or Pso diagnosis date (i.e., index date): 1) topical or ultraviolet (UV) therapy only, 2) conventional immunomodulating drugs, and 3) biologics. In the Pso and PsA groups, we calculated the proportion of each treatment groups at baseline and the proportion of a specific conventional or biologic immunomodulating agent over time.
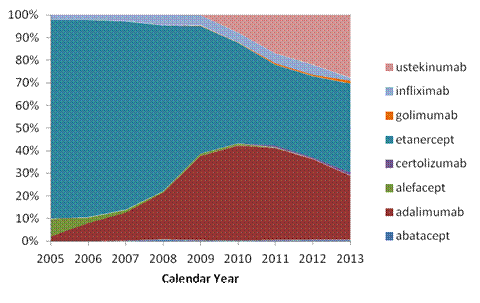
Results: We included 49,950 Pso and 9,706 PsA patients. Mean age (SD) was 46.9 (13.3) years for Pso and 48.2 (11.1) years for PsA. Sixty percent of patients with PsA also had psoriasis. Overall, for baseline treatment of Pso, 82.8% used topical or UV therapy only, 6.8% had conventional immunomodulating drugs and 10.4% used biologic drugs. Baseline treatment of PsA included 18.0% topical or UV therapy only, 32.2% conventional immunomodulating drugs and 49.8% biologic drugs. No substantial time trends were noted in either group. Methotrexate was the most commonly used conventional immunomodulating drug in both Pso (48.7% in 2005 to 61.5% in 2013) and PsA (83.2% in 2005 to 74.2% in 2013) groups. For Pso, etanercept was the most commonly used biologic drug, albeit with a decreasing trend (87.9% to 39.4%), and adalimumab was the 2nd most commonly used drug (2.2% to 28.4%) from 2005 to 2013. Ustekinumab use in Pso has increased from 0.2% in 2009 to 27.7% in 2015 (Figure 1). Among biologic drug users for PsA, etanercept, adalimumab and infliximab were the three most commonly used agents across all years. Ustekinumab use in PsA has increased from 0.3% in 2009 to 6.5% in 2013 (Figure 2).
Conclusion: Over the past decade, we noted a substantial trend in the patterns of biologic treatment for both Pso and/or PsA. The change in the use of different biologic drugs among patients with Pso has been more pronounced over time compared to PsA, most likely related to the availability of ustekinumab.
Figure 1. Trend in the use of biologic drugs in psoriasis
Figure 2. Trend in the use of biologic drugs in psoriatic arthritis
To cite this abstract in AMA style:
Merola JF, Lii J, Desai RJ, Solomon DH, Kim SC. Secular Trends in Treatment Patterns for Psoriasis and Psoriatic Arthritis: A Population-Based Cohort Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/secular-trends-in-treatment-patterns-for-psoriasis-and-psoriatic-arthritis-a-population-based-cohort-study/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secular-trends-in-treatment-patterns-for-psoriasis-and-psoriatic-arthritis-a-population-based-cohort-study/