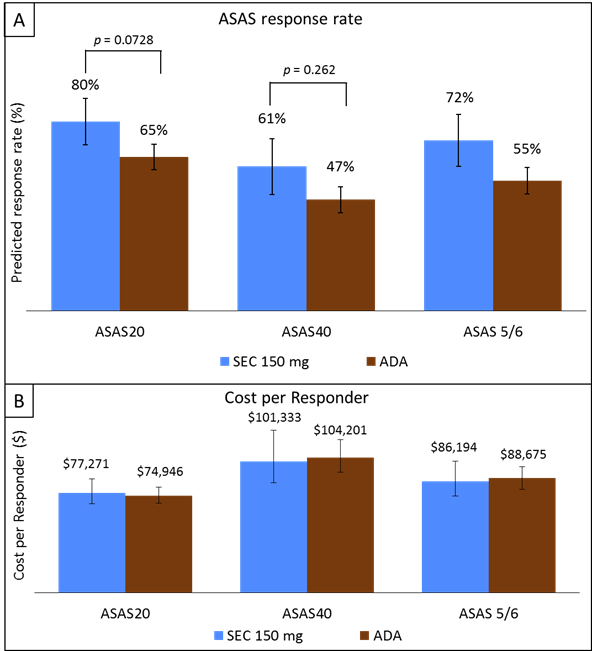Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Jessica Walsh1, Efthalia Nikoglou2, Praveen Gunda3, Yujin Park4, Steffen Jugl5
1 School of Medicine, University of Utah, Salt Lake City, UT, USA
2 Novartis Ireland, Ltd, Dublin, Ireland
3 Novartis Healthcare Pvt. Ltd., Hyderabad, India
4 Novartis Pharmaceuticals Corporation, East Hanover, NJ, USA
5 Novartis Pharma AG, Basel, Switzerland
Background/Purpose: The cost per responder analysis attempts to quantify the relative value of the two comparator drugs by assessing how the two agents compare in terms of cost per treatment outcome. The objective of this analysis was to estimate and compare the long-term cost per responder based on the Assessment of Spondyloarthritis International Society (ASAS) outcomes following 52 weeks of treatment of ankylosing spondylitis (AS) with the anti-IL-17A antibody Secukinumab relative to the anti-TNF antibody Adalimumab.
Methods: The cost per responder for each treatment namely Secukinumab and Adalimumab was estimated by dividing the drug acquisition cost for the course of treatment with its response rate. Drug costs were estimated on the basis of the official US drug acquisition costs and the number of doses required for 52 weeks. Long-term response rates were estimated using a matching-adjusted indirect comparison (MAIC) technique based on the data from MEASURE 2 and ATLAS clinical trials of Secukinumab and Adalimumab, respectively. MAIC analysis matched the age, gender distribution, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), C-reactive protein (CRP) and TNF-naive proportion at the baseline. A sensitivity analyses was also conducted by varying the choice of baseline characteristics (all above except BASDAI) used in MAIC analysis.
Results: MAIC analysis showed that ASAS (20, 40 and 5/6) response rates were significantly higher for Secukinumab compared to Adalimumab at 52 weeks. ASAS 20, ASAS 40 and ASAS 5/6 response rates were 80% vs 65%, 61% vs 47%, 72% vs 55% for Secukinumab vs Adalimumab, respectively (Figure 1A). The cost per ASAS 20 responder was $77,271 vs $74,946, cost per ASAS40 responder was $101,333 vs $104,202, whereas, costs per ASAS 5/6 responder was $86,194 vs $88,675 for Secukinumab vs Adalimumab, respectively (Figure 1B). While the costs per ASAS (40 and 5/6) responders were about 3% lower for Secukinumab compared to Adalimumab, the cost per ASAS 20 responders for Secukinumab was about 3% higher than that of Adalimumab at 52 weeks. Sensitivity analyses for ASAS response rates and cost per responder showed similar results, confirming the robustness of our main analysis.
Conclusion: The long-term cost per responder for ASAS (40 and 5/6) outcomes over 52-week treatment period were lower for Secukinumab compared to Adalimumab in AS patients. These findings indicate that Secukinumab represents a cost-efficient treatment choice for the treatment of AS patients in the US. Keywords: Adalimumab, Ankylosing spondylitis, Cost per responder, Matching-adjusted indirect comparison, Secukinumab
Figure 1: Predicted response rate (A) and cost per responder (B) analysis of ASAS (20, 40 and 5/6) for Secukinumab vs Adalimumab for the treatment of ankylosing spondylitis at 52 weeks in principal analysis
To cite this abstract in AMA style:
Walsh J, Nikoglou E, Praveen G, Park Y, Jugl S. Secukinumab Vs Adalimumab for the Treatment of Ankylosing Spondylitis: A Cost per Responder Analysis at 52 Weeks from the US Perspective [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/secukinumab-vs-adalimumab-for-the-treatment-of-ankylosing-spondylitis-a-cost-per-responder-analysis-at-52-weeks-from-the-us-perspective/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-vs-adalimumab-for-the-treatment-of-ankylosing-spondylitis-a-cost-per-responder-analysis-at-52-weeks-from-the-us-perspective/