Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Power Doppler (PD) ultrasonography (PDUS) is a sensitive non-invasive imaging technology used to assess joint synovitis and enthesitis of psoriatic arthritis (PsA) in clinical practice.1,2 The European League Against Rheumatism and the Outcome Measures in Rheumatology (EULAR-OMERACT) developed a standardized and sensitive to change ultrasonography composite scoring system (the EULAR-OMERACT global synovitis score [GLOESS]) to detect and score joint synovitis.3 Here we report primary (12-week) efficacy and safety results from the ULTIMATE study (NCT02662985), the first large, randomized, double-blind, placebo-controlled phase III study, primarily designed to assess the time course of response to subcutaneous secukinumab on joint synovitis with PDUS in patients with PsA.
Methods: This is a 52-week study with a 12-week double-blind treatment period (TP 1) followed by 12-week open-label (TP 2) and 6-month open-label extension (TP 3). The study enrolled biologic-naïve patients with active PsA and inadequate response to conventional DMARD(s), with joint synovitis on PDUS (at least one joint [out of 48] with both total synovitis PDUS score and PD signal ≥2; or at least two joints with PDUS score ≥2 and PD signal ≥1) at screening and baseline and at least one clinical enthesitis site at baseline. Patients were randomized (1:1) to receive either secukinumab (300 or 150 mg according to severity of skin disease) or placebo weekly followed by 4-weekly dosing starting at Week 4. The primary endpoint was GLOESS, difference in mean change from baseline to Week 12 between secukinumab and placebo determined with mixed-effect model repeated measures analysis. Key secondary endpoints included proportion of patients with ACR20 and ACR50 responses at Week 12 and change in Spondyloarthritis Research Consortium of Canada (SPARCC) enthesitis index from baseline to Week 12. Safety analyses included all patients who received at least 1 dose of study treatment.
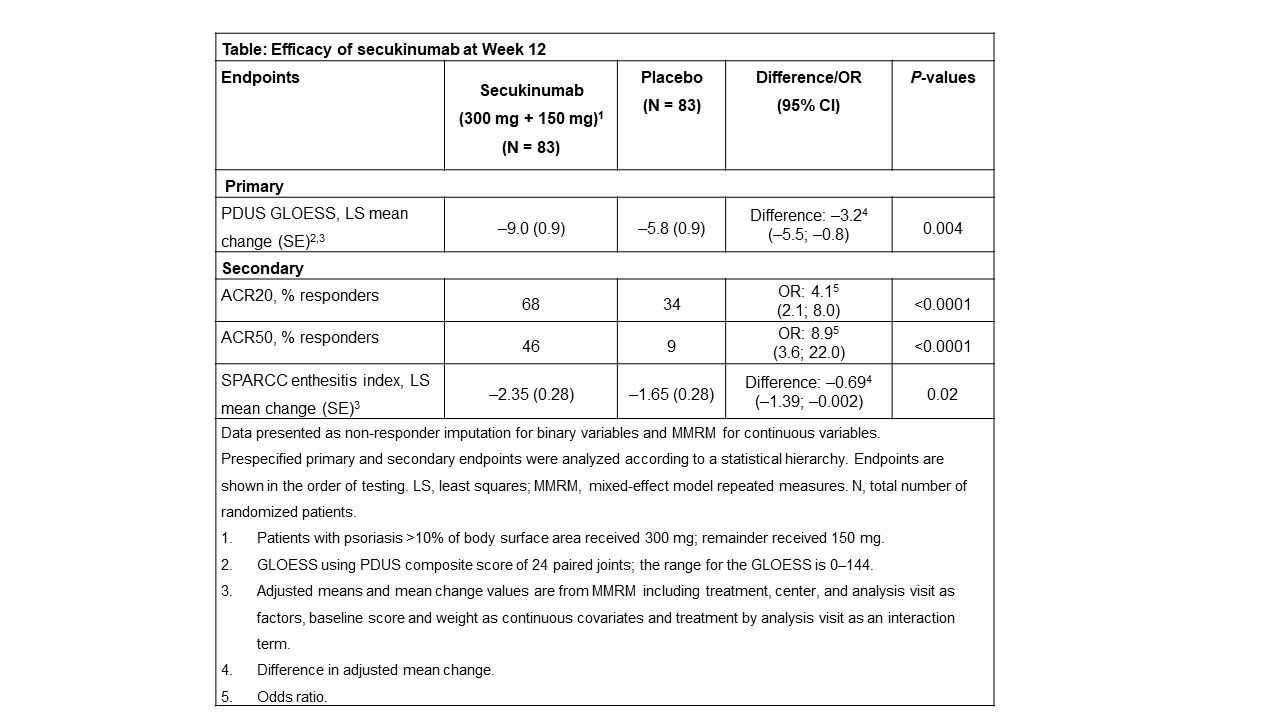
Results: Of 166 patients enrolled, 96% (160/166) completed 12-weeks treatment (secukinumab: 99% [82/83] and placebo: 94% [78/83]). Demographics and baseline clinical characteristics were comparable across groups; mean (SD) GLOESS was 24 (16) for secukinumab and 27 (17) for placebo. Mean baseline SPARCC enthesitis index was 4.2 (2.9) for secukinumab and 4.4 (3.3) for placebo. The primary endpoint was met; adjusted mean change in GLOESS was significantly higher with secukinumab vs placebo (–9.0 vs –5.8; P = 0.004) at Week 12 (Table), with statistical significance observed as early as Week 1. All key secondary endpoints were met (Table). No new or unexpected safety signals were reported.
Conclusion: Secukinumab demonstrated rapid and significant decrease in synovitis in a period of 12 weeks, as assessed by GLOESS in biologic-naïve patients with active PsA. Secukinumab also demonstrated superior efficacy on ACR20/50 responses and on SPARCC enthesitis vs placebo at Week 12. The safety profile of secukinumab was consistent with previous reports.
References: 1. D’Agostino MA and Coates LC. J Rheumatol 2019;46:337–39; 2. Uson J, et al. Rheumatol Clin 2018;14:27–35; 3. D’Agostino MA, et al. RMD Open 2017;3:e000428.
 Efficacy of secukinumab at Week 12
Efficacy of secukinumab at Week 12
To cite this abstract in AMA style:
D’Agostino M, Schett G, López-Rdz A, Šenolt L, Maldonado-Cocco J, Burgos-Vargas R, Naredo E, Carron P, Boers M, Duggan A, Goyanka P, Gaillez C. Secukinumab Significantly Decreased Joint Synovitis Measured by Power Doppler Ultrasonography in Biologic-naïve Patients with Active Psoriatic Arthritis: Primary (12-Week) Results from a Randomized, Placebo-Controlled Phase III Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/secukinumab-significantly-decreased-joint-synovitis-measured-by-power-doppler-ultrasonography-in-biologic-naive-patients-with-active-psoriatic-arthritis-primary-12-week-results-from-a-randomized-p/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-significantly-decreased-joint-synovitis-measured-by-power-doppler-ultrasonography-in-biologic-naive-patients-with-active-psoriatic-arthritis-primary-12-week-results-from-a-randomized-p/
