Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
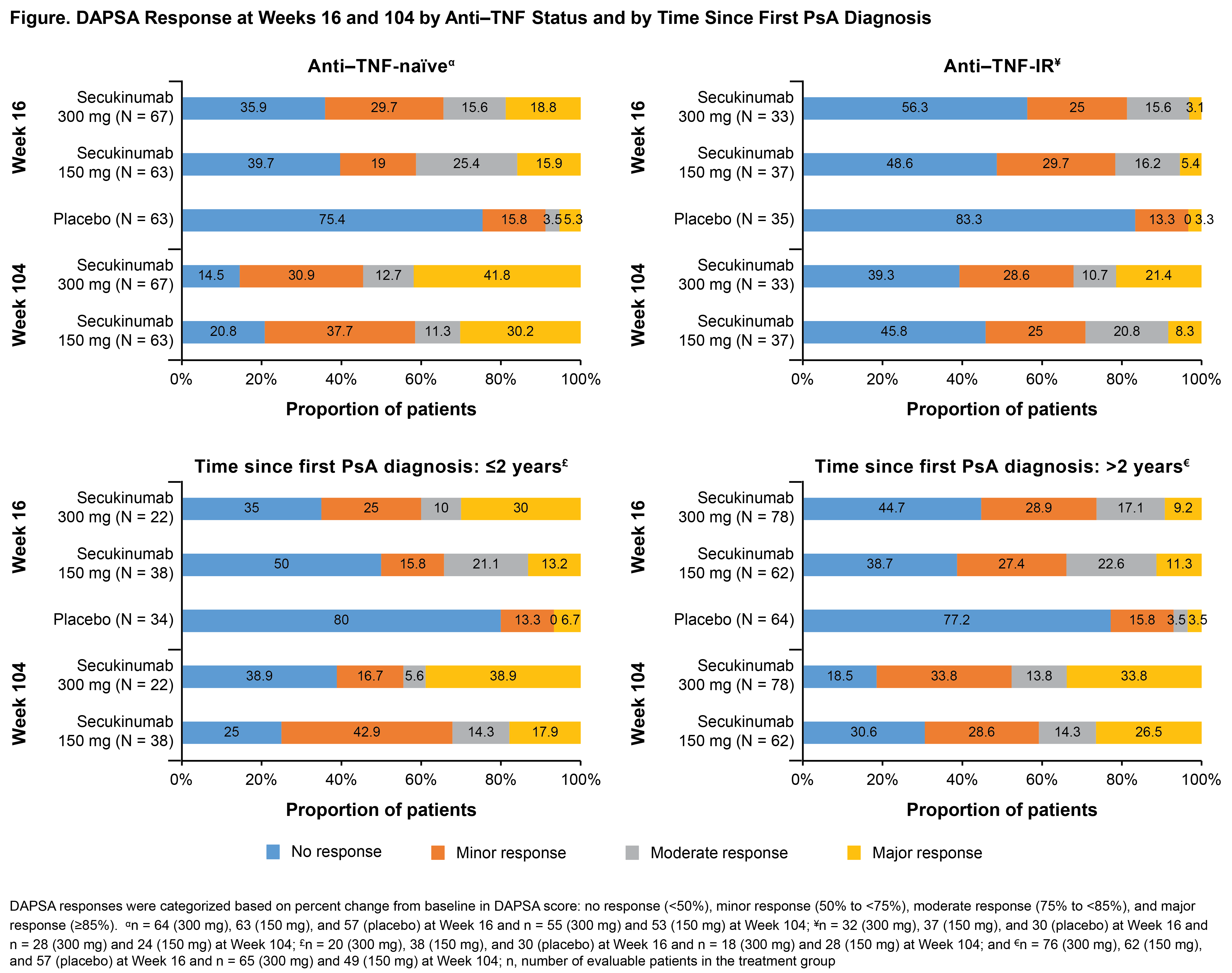
Background/Purpose: DAPSA score is a validated tool to measure disease activity states and response criteria, focusing on peripheral joint involvement in patients (pts) with PsA.1 Secukinumab, a fully human anti–IL-17A mAb, significantly improved signs and symptoms vs placebo (PBO) at Week (Wk) 24, with sustained ACR responses through Wk 104 from the FUTURE 2 study2,3. This post-hoc analysis assessed DAPSA responses through Wk 104 from FUTURE 2 study.
Methods: FUTURE 2 study design has been previously reported.3 DAPSA score was derived as the sum of five variables: tender and swollen joint counts (TJC 68 and SJC 66), pt global and pt pain assessed on a 10 cm visual analog scale, and CRP level (mg/dL). DAPSA responses are presented for secukinumab 300 and 150 mg (approved doses) in overall population and in pts stratified by prior anti-TNF therapy use (anti–TNF-naïve vs inadequate response/intolerance to these agents [anti–TNF-IR]) and time since first PsA diagnosis (≤2 vs >2 years) using observed data.
Results: Baseline demographics and clinical characteristics were similar across treatment groups.3 A total of 96,100, and 87 pts treated with secukinumab 300, 150 mg, and PBO, respectively, were available for the evaluation of DAPSA response at Wk 16. DAPSA response in overall population showed moderate/major response in 16%/14% and 22%/12% vs 2%/5%; minor response in 28% and 23% vs 15%; no response in 43% and 43% vs 78% in secukinumab 300 and 150 mg vs PBO, respectively. DAPSA response rates were higher and sustained at Wk 104 in secukinumab-treated pts. A higher proportion (35% and 23%) of pts showed major response, with moderate response observed in 12% and 14% of pts treated with secukinumab 300 and 150 mg, respectively, at Wk 104. The proportion of pts achieving DAPSA response at Wks 16 and 104 by anti-TNF use and time since first PsA diagnosis is shown in the figure. The proportion of pts in overall population meeting DAPSA core components thresholds and other components of PsA among pts with DAPSA moderate/major response at Wks 16 and 104 is shown in the table.
Conclusion: In the overall population, around 30% of secukinumab-treated pts at Wk 16 achieved DAPSA moderate/major response vs <5% in PBO group, with numerical increase in the major response at Wk 104. Moderate/major response at Wk 16 was observed in pts regardless of prior anti-TNF use or time since first PsA diagnosis. Majority of the pts who achieved major response met all core components criteria, in contrast to the pts with moderate response.
References: 1. Smolen JS, et al. Clin Exp Rheumatol. 2015; 33: S48–50; 2. McInnes IB, et al. Arthritis Rheumatol. 2016;68 (suppl 10); 3. McInnes IB, et al. Lancet 2015;386:1137–46.
|
Table: Proportion of Patients Meeting the Selected Variable Thresholds |
|||||||
|
|
DAPSA moderate response |
DAPSA major response |
|||||
|
Criterion, n/M (%) |
Week |
Secukinumab 300 mg |
Secukinumab 150 mg |
Placebo |
Secukinumab 300 mg |
Secukinumab 150 mg |
Placebo |
|
PtGA <1 cm
|
16 |
3/15 (20.0) |
5/22 (22.7) |
2/2 (100.0) |
7/13 (53.8) |
7/12 (58.3) |
1/4 (25.0) |
|
104 |
1/10 (10.0) |
1/11 (9.1) |
– |
16/29 (55.2) |
7/18 (38.9) |
– |
|
|
CRP <5 mg/dL |
16 |
15/15 (100.0) |
22/22 (100.0) |
2/2 (100.0) |
13/13 (100.0) |
12/12 (100.0) |
4/4 (100.0) |
|
104 |
10/10 (100.0) |
11/11 (100.0) |
– |
29/29 (100.0) |
18/18 (100.0) |
– |
|
|
SJC 66 ≤1
|
16 |
7/15 (46.7) |
14/22 (63.6) |
2/2 (100.0) |
11/13 (84.6) |
11/12 (91.7) |
4/4 (100.0) |
|
104 |
8/10 (80.0) |
7/11 (63.6) |
– |
28/29 (96.6) |
18/18 (100.0) |
– |
|
|
TJC 68 ≤1
|
16 |
5/15 (33.3) |
13/22 (59.1) |
0 |
13/13 (100.0) |
11/12 (91.7) |
4/4 (100.0) |
|
104 |
7/10 (70.0) |
4/11 (36.4) |
– |
27/29 (93.1) |
15/18 (83.3) |
– |
|
|
HAQ-DI ≤0.5 |
16 |
13/15 (86.7) |
12/22 (54.5) |
2/2 (100.0) |
10/13 (76.9) |
8/12 (66.7) |
3/4 (75.0) |
|
104 |
8/10 (80.0) |
6/11 (54.5) |
– |
26/29 (89.7) |
15/18 (83.3) |
– |
|
|
Enthesitis resolution |
16 |
13/15 (86.7) |
21/22 (95.5) |
2/2 (100.0) |
13/13 (100.0) |
11/12 (91.7) |
4/4 (100.0) |
|
104 |
9/10 (90.0) |
9/11 (81.8) |
– |
27/29 (93.1) |
15/18 (83.3) |
– |
|
|
Dactylitic resolution |
16 |
12/15 (80.0) |
20/22 (90.9) |
2/2 (100.0) |
12/13 (92.3) |
10/12 (83.3) |
3/4 (75.0) |
|
104 |
9/10 (90.0) |
11/11 (100.0) |
– |
25/29 (86.2) |
17/18 (94.4) |
– |
|
|
PASI Score ≤1 |
16 |
14/15 (93.3) |
14/22 (63.6) |
1/2 (50.0) |
12/13 (92.3) |
8/12 (66.7) |
4/4 (100.0) |
|
104 |
8/10 (80.0) |
7/11 (63.6) |
– |
26/29 (89.7) |
11/18 (61.1) |
– |
|
|
CRP, C-reactive protein; DAPSA, Disease Activity Index for PSoriatic Arthritis; HAQ-DI, Health Assessment Questionnaire – Disability Index; M, the number of evaluable patients; n, number of patients who met the threshold criterion; PASI, Psoriasis Area and Severity Index; PtGA, Patient Global Assessment; SJC, swollen joint count; TJC, tender joint count |
|||||||
To cite this abstract in AMA style:
Smolen JS, Mease PJ, Ritchlin CT, Kvien TK, Pricop L, Fox T, Rasouliyan L, Jugl S, Gaillez C. Secukinumab Provides Sustained Improvement in Major and Moderate Response of Disease Activity Index for Psoriatic Arthritis (DAPSA): 2-Year Results from a Phase 3 Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/secukinumab-provides-sustained-improvement-in-major-and-moderate-response-of-disease-activity-index-for-psoriatic-arthritis-dapsa-2-year-results-from-a-phase-3-study/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-provides-sustained-improvement-in-major-and-moderate-response-of-disease-activity-index-for-psoriatic-arthritis-dapsa-2-year-results-from-a-phase-3-study/