Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Nail psoriasis (PsO) is present in up to 80% in psoriatic arthritis (PsA) patients (pts) and associated with significant pain, psychosocial disability, decreased physical function and quality of life (QoL).1 Nail PsO is considered as one of the six core PsA domains by GRAPPA2 and is a predictor of severe disease with joint involvement and structural damage. Secukinumab (SEC) has demonstrated efficacy for pts with nail PsO in the TRANSFIGURE study3 and improvement in signs and symptoms and low radiographic progression in pts with PsA in FUTURE 5 (NCT02404350) study4.Here, we report the efficacy of SEC on nail PsO, and other facets of disease, including radiographic progression, in the nail subset through 52 weeks (wks) from the FUTURE 5 study.
Methods: Pts (N=996) with active PsA, were randomized to subcutaneous SEC 300 mg loading dosage (LD; 300 mg), 150 mg LD (150 mg), 150 mg no LD or placebo (PBO). All groups received SEC or PBO at baseline (BL), Wks 1, 2, 3, and 4, and then every 4 wks. Efficacy assessments through Wk 52 included mNAPSI, radiographic progression (mTSS), ACR, PASI, HAQ-DI, SF-36 PCS, PsAQoL and resolution of dactylitis and enthesitis. Analyses through Wk 16 used non-responder imputation (NRI) for binary and mixed-effect model repeated measure (MMRM) for continuous variables. Observed data are presented for radiographic progression at Wks 24 and 52, and for all efficacy endpoints at Wk 52.
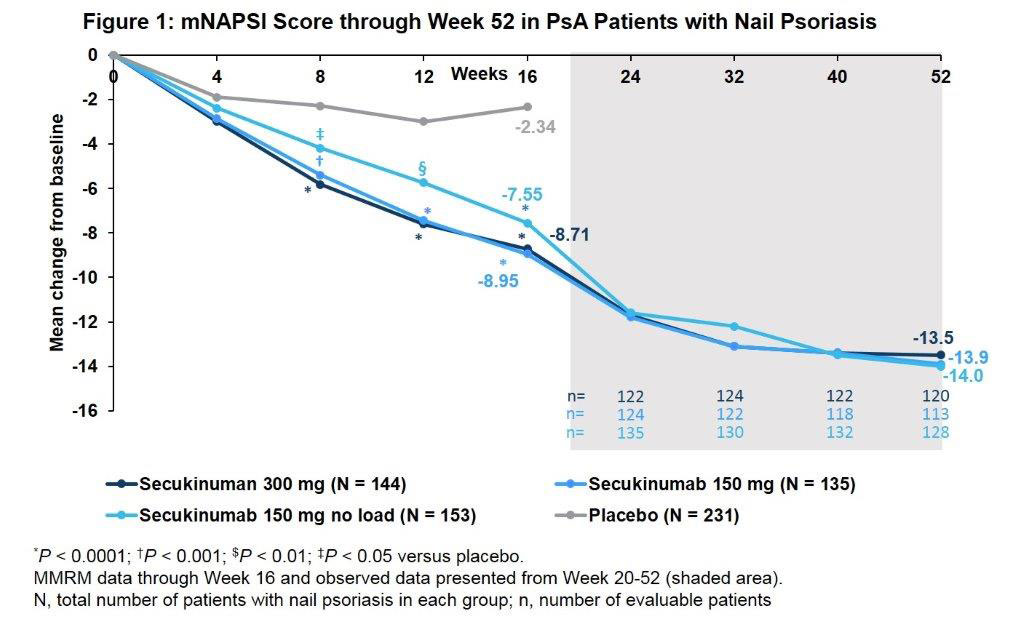
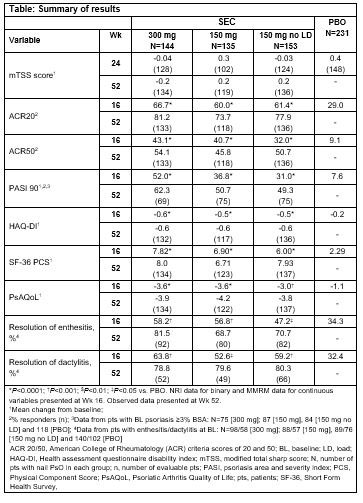
Results: A total of 663/996 (66.6%) PsA pts had concomitant nail PsO at BL. Demographics and BL disease characteristics were balanced between treatment groups in the nail subset, which were comparable with overall population. The total mean Nail Psoriasis Severity Index (mNAPSI) score at BL was 16.4. SEC 300 and 150 mg doses improved nail PsO vs. placebo (PBO) at Wk 8, 12 and 16 (P < 0.0001), with further improvements through Wk 52 (Figure). Mean change from baseline in mTSS score at Wks 24 and 52 are shown in the Table. Proportions of pts with no radiographic progression (change from BL in mTSS ≤0.5) with SEC at 52 Wks were 94.0% (300 mg LD), 83.5% (150 mg LD), and 88.4% (150 mg No LD). ACR20/50 and PASI 90 responses, resolution of dactylitis and enthesitis, physical function and QoL were also improved with SEC vs. PBO at Wk 16 with sustained improvements through 52 wks (Table).
Conclusion: Secukinumab provided sustained improvements in nail disease, signs and symptoms of PsA, physical function and QoL along with low radiographic progression through 52 wks in PsA pts with moderate to severe nail PsO.
References:
- Baran R. Dermatology 2010;221(Suppl1):1-5.
- Coates LC, et al. Arthritis Rheumatol. 2016;68:1060-71.
- Reich K, et al. Br J Dermatol. 2018. doi: 10.1111/bjd.17351.
- Mease PJ et al., Arthritis Rheumatol. 2018; 70 (suppl 10).
To cite this abstract in AMA style:
Nash P, Mease P, Kirkham B, Balsa A, Singhal A, Quebe-Fehling E, Pricop L, Gaillez C. Secukinumab Provides Improvement in Nail Psoriasis and Inhibition of Radiographic Progression in Psoriatic Arthritis Patients with Nail Phenotype: 52-Week Results from a Phase III Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/secukinumab-provides-improvement-in-nail-psoriasis-and-inhibition-of-radiographic-progression-in-psoriatic-arthritis-patients-with-nail-phenotype-52-week-results-from-a-phase-iii-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/secukinumab-provides-improvement-in-nail-psoriasis-and-inhibition-of-radiographic-progression-in-psoriatic-arthritis-patients-with-nail-phenotype-52-week-results-from-a-phase-iii-study/