Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Hepatitis B virus (HBV) screening is insufficiently completed prior to initiation of immunosuppressive therapy nationwide. Various factors including alert fatigue, provider mistakes, non-compliance, and EMR faults / errors all play a role in leading to insufficient screening. HBV status is often difficult to ascertain given the indolent and asymptomatic nature of HBV progression. Immunosuppression can lead to HBV reactivation. Concerningly, the prevalence of HBV is higher in veterans than the general population at up to 1.0%, highlighting the increased importance of screening within this specific population (1). Additionally, prevalence is also higher within the Black American population as compared to others. While HBV screening rates within various Veterans Affairs Medical Centers (VAMC) facilities have been studied with regards to prescribing Rituximab, which likely has the highest risk of reactivation, less research has been conducted to analyze screening rates before start of a wider array of anti-rheumatic medications, highlighting the need for further research.
Methods: A five-year retrospective study was performed reviewing HBV serologies – including HBV core Ab, surface Ag, and HBV DNA in patients who were started on immunosuppressive therapy within our rheumatology clinic at the VAMC in Memphis, TN. Using collected data from the pharmacy, patients were extracted who had previously been prescribed csDMARDs, bDMARD, and small molecules. The study period was set from 1/1/2017 to 12/31/2022. To more directly identify how well we were screening patients with a statistically higher risk of HBV infection, past or present, Black Americans within our clinic were specifically studied, with plans to study data from other races in the future as a comparison.
Results: Within our study period, 551 instances were found in which Black American patients had been prescribed medications from the list above. Out of these 551 patients, 75.9% had HBV screening labs prior to initiation of DMARD therapy. However, only 32.7% of patients had screening labs within six months of starting treatment. For those that did not have labs checked prior to start of treatment, 33.7% of these patients had labs checked within six months after starting treatment. Out of our population, 11.2% were HBV core Ab positive. Of these patients, 79% were sent to Hepatology and 50% were started on antiviral prophylaxis. No episodes of HBV reactivation leading to fulminant hepatitis or clinical deterioration / death were identified.
Conclusion: Our findings suggest a large number of patients are not being appropriately screened prior to initiation of DMARDs. While no episodes of HBV reactivation leading to fulminant hepatitis or clinical deterioration / death were identified, further correction of this lack of pre-screening is critical to ensure safety of our patients, particularly within this veteran group who inherently are at a higher risk. We plan to study data from other races to determine if there are any statistical differences between the two groups to further help us implement quality initiatives to improve outcomes.References 1. Garren P, Serper M. Chronic Hepatitis B in US Veterans. Curr Hepatol Rep. 2019;18(3):310-315.
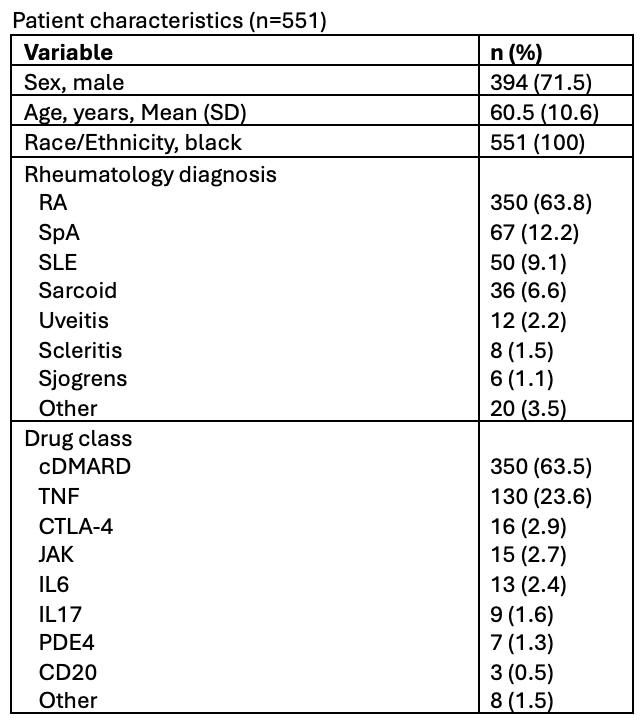 Table 1: Patient characteristics
Table 1: Patient characteristics
.jpg) Table 2: Screening and prescription outcomes within diagnoses
Table 2: Screening and prescription outcomes within diagnoses
.jpg) Table 3: Screening and prescription outcomes within drug class
Table 3: Screening and prescription outcomes within drug class
To cite this abstract in AMA style:
Austin D, Zulfiqar B, Adla A, Zuber J, Sullivan J. Screening for Hepatitis B in a Veterans Health Administration Subpopulation with Rheumatological Disease Prior to Initiating Immunosuppressive Therapy: a retrospective study on testing and treatment within the Black American population from the VAMC in Memphis, TN [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/screening-for-hepatitis-b-in-a-veterans-health-administration-subpopulation-with-rheumatological-disease-prior-to-initiating-immunosuppressive-therapy-a-retrospective-study-on-testing-and-treatment-w/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/screening-for-hepatitis-b-in-a-veterans-health-administration-subpopulation-with-rheumatological-disease-prior-to-initiating-immunosuppressive-therapy-a-retrospective-study-on-testing-and-treatment-w/
