Session Information
Date: Sunday, November 10, 2019
Title: Measures Of Healthcare Quality Poster I: Testing, Screening, & Treating
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Background/Purpose: Tofacitinib and baricitinib (JAK inhibitors) are agents used for rheumatoid arthritis (RA), seronegative spondyloarthropathy (SpA), and juvenile idiopathic arthritis (JIA). Tocilizumab and sarilumab are biologics (IL6 inhibitors) for the management of inflammatory arthritis, and giant cell arteritis. Aforementioned drugs elevate total cholesterol and LDL levels. Primary aim is to assess compliance for screening and treatment per guidelines of the American College of Rheumatology (ACR) (ARP Practice Committee, 2017) for hyperlipidemia in patients receiving tofacitinib, tocilizumab, sarilumab, and baricitinib at Lehigh Valley Health Network (LVHN).
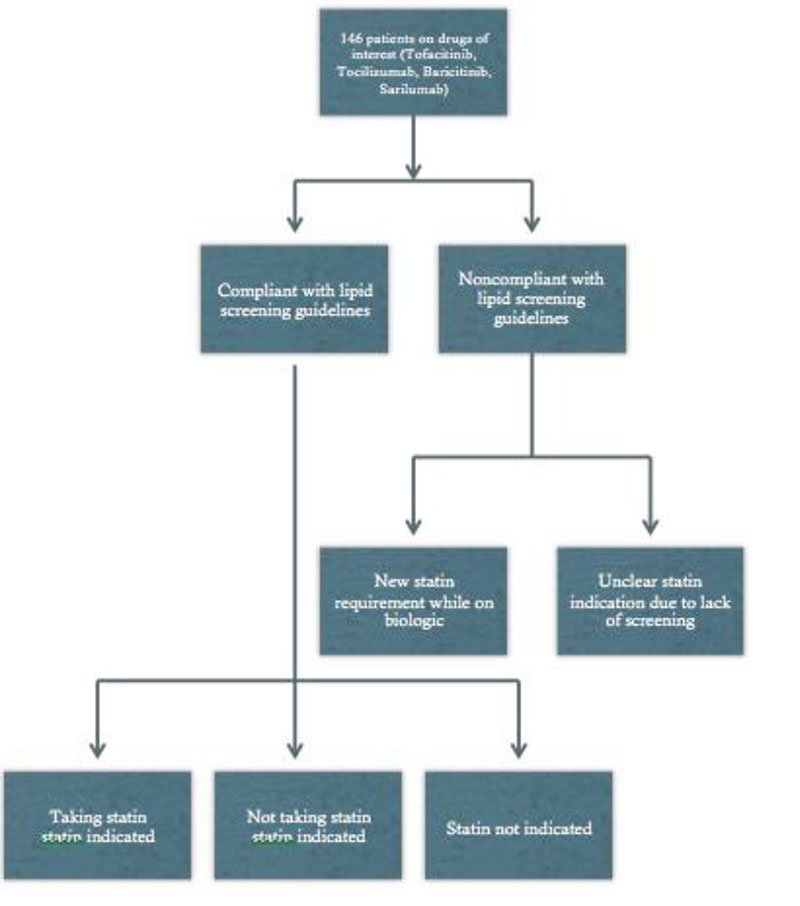
Methods: Data reviewed on 146 patients within LVHN, from December 2018 to April 2018. Subjects were retrieved from Epic by searching for orders for the drugs of interest. Manual Chart Review was utilized to determine: age, gender, ethnicity, pathology, medication, steroid dose, additional DMARDs, medication duration, date of drug initiation, previous statin, baseline lipid panel, frequency of lipid screenings, and statin initiated.
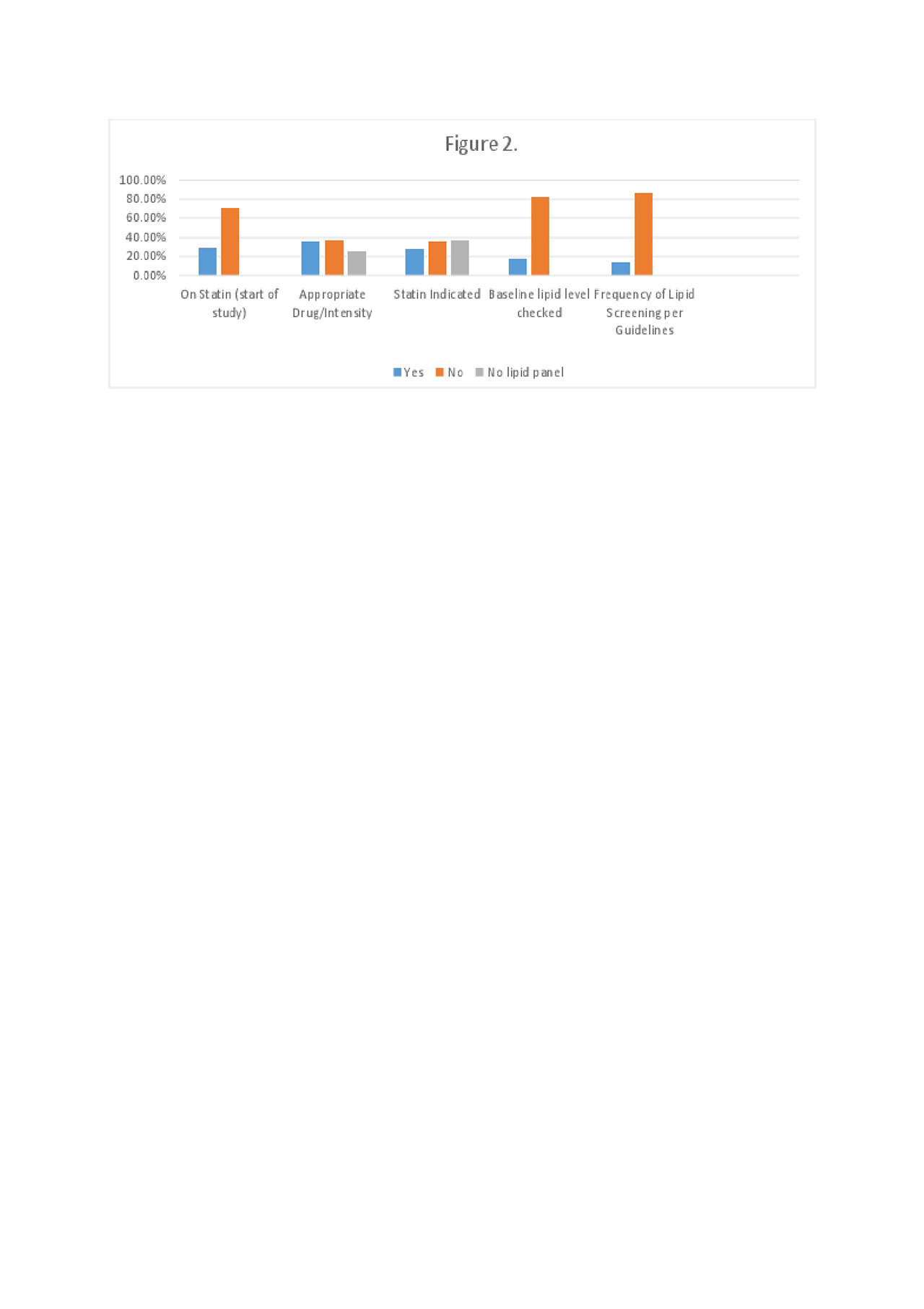
Results: Table 1 shows demographic and clinical variables of the charts reviewed. Medications used were the following: tofacitinib (64%), tocilizumab (27%), sarilumab (8%), and baricitinib (0.68%). Patients examined were afflicted with the following disease processes: RA (90%), GCA (4%), SpA (4%), and JIA (2%). Prior to the intervention 29% of patients were on a statin, and 28% had indications for a statin yet were not prescribed one. Additionally, only 17% of patients had a proper baseline lipid screening 4-8 weeks after starting the drug. Only 13% of patient had lipid screenings every 6 months after institution of the drug. Mean age was 56 years old, with 77% female, and average duration on drug of interest was 760.5 days.
Conclusion: According to ACR guidelines, laboratory monitoring for patients on JAK and IL6 inhibition is recommended due to treatment related changes in neutrophils, platelets, lipids, and liver specific enzymes (ARP Practice Committee, 2017). Additionally, British Society for Rheumatology (BSR) advocates for baseline lipid screenings, and rescreening every 3 months (Malaviya, 2014). After analyzing patients in LVHN there is room for improvement. Twenty eight percent of patients in the project have an indication for statin therapy, and are not being treated. Eighty two percent of patients lacked baseline lipid screening 4-8 weeks after starting JAK and IL6 inhibition. An intervention educating rheumatology providers on guidelines for these medications, and options for managing subjects with lipid lowering agents will follow initial data assessment. Data will be re-collected and assessed for improvement in guideline compliance and treatment following the intervention. After the intervention we expect to see increased compliance with lipid screening and management with patients on the drugs of interest within LVHN.
To cite this abstract in AMA style:
Torelli W, Ross J, Quinn T, Erickson K, Soliman A, Harit A. Screening and Treating Hyperlipidemia in Patient’s on Tofacitinib, Tocilizumab, Sarilumab, and Baricitinib [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/screening-and-treating-hyperlipidemia-in-patients-on-tofacitinib-tocilizumab-sarilumab-and-baricitinib/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/screening-and-treating-hyperlipidemia-in-patients-on-tofacitinib-tocilizumab-sarilumab-and-baricitinib/