Session Information
Date: Sunday, November 8, 2015
Title: Systemic Lupus Erythematosus - Clinical Aspects and Treatment Poster Session I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Apoptosis is needed to eliminate
auto-reactive T and B cells during immune responses; failure of elimination is important
in development of systemic lupus erythematosus (SLE). The BCL-2 family helps
regulate immune cell homeostasis; elevated Bcl-2 expression may contribute to cell
death abnormalities for auto-reactive cells. Venetoclax (ABT-199), a selective,
small-molecule BCL-2 inhibitor, was examined in a phase 1 study of safety, tolerability,
pharmacokinetics, and pharmacodynamics (PD) in female patients (pts) with SLE.
Methods: This was a single and multiple ascending
dose, double-blind, randomized, placebo-controlled study (NCT01686555) in women
aged 18–65 y with SLE for >6 mo, receiving stable SLE therapy. Twelve
cohorts were planned (N=96); in each cohort, 6 pts received venetoclax and 2
received placebo. Pts in cohorts 1–6 received single doses of venetoclax 10,
30, 90, 180, 300, and 500 mg, respectively. Pts in the multiple ascending dose
arms (cohorts 7–12) received 2 cycles (once daily for 1 wk, then 3 wk off in
each cycle) of venetoclax 30, 60, 120, 240, 400, and 600 mg, beginning after safety
evaluation of the single-dose venetoclax 90-mg cohort.
Results: In 98 enrolled pts, common adverse events
(AEs) were headache and gastrointestinal disorders (diarrhea, nausea, vomiting;
Table). Most drug-related AEs were mild to moderate; no serious AE was
reported with venetoclax. There were no deaths and no clinically relevant
abnormalities of vital signs or electrocardiograms. Exposure to venetoclax was
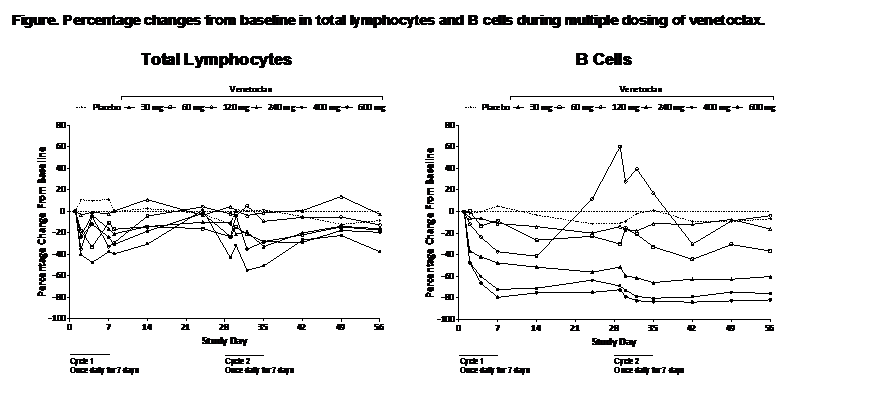
dose proportional, with a half-life of ~7–17 h after single doses. PD effects
of Bcl-2 inhibition included dose-dependent reductions of total lymphocytes and
subsets (Figure) after single and multiple venetoclax doses up to 600 mg.
B cells were most sensitive to Bcl-2 inhibition, with >80% reduction from
baseline in high-dose groups. For lymphocytes and T cells, changes were less marked
with multiple dosing, and recovery of total lymphocytes occurred
during the dosing interval of cyclic multiple dose regimens (Figure). Compared
with lymphocytes and subsets, levels of neutrophils, platelets, reticulocytes,
hemoglobin, and safety laboratory values showed no consistent patterns or marked
changes across single- and multiple-dosing groups.
Conclusion: In female SLE pts, venetoclax was well
tolerated in single (10–500 mg) and multiple (30–600 mg) doses; exposure
appeared consistent with findings in cancer pts. PD assessments demonstrated specific
reductions of lymphocytes and subsets by inhibition of BCL-2, as expected from preclinical
and ex vivo findings.
|
Table. Adverse Events (AEs) in Patients Who Received Venetoclax |
||||||||||||||
|
AE, n (%) |
Single Doses, mg |
Multiple Doses, mg |
||||||||||||
|
10 (n=6) |
30 (n=6) |
90 (n=6) |
180 (n=6) |
300 (n=6) |
500 (n=6) |
Placebo (n=12) |
30 (n=6) |
60 (n=6) |
120 (n=6) |
240 (n=6) |
400 (n=6) |
600 (n=7) |
Placebo (n=13) |
|
|
Any |
5 (83.3) |
6 (100) |
4 (66.7) |
3 (50.0) |
2 (33.3) |
2 (33.3) |
6 (50.0) |
5 (83.3) |
4 (66.7) |
2 (33.3) |
3 (50.0) |
5 (83.3) |
4 (57.1) |
6 (46.2) |
|
Study drug-related |
2 (33.3) |
4 (66.7) |
3 (50.0) |
0 |
0 |
2 (33.3) |
4 (33.3) |
2 (33.3) |
3 (50.0) |
2 (33.3) |
1 (16.7) |
2 (33.3) |
3 (42.9) |
4 (30.8) |
|
Severe |
0 |
0 |
1 (16.7) |
0 |
0 |
0 |
0 |
0 |
1 (16.7) |
0 |
0 |
0 |
0 |
0 |
|
Serious |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 (7.7) |
|
Headache |
1 (16.7) |
2 (33.3) |
1 (16.7) |
2 (33.3) |
0 |
2 (33.3) |
3 (25.0) |
2 (33.3) |
3 (50.0) |
1 (16.7) |
0 |
2 (33.3) |
1 (14.3) |
3 (23.1) |
|
Diarrhea |
0 |
2 (33.3) |
0 |
1 (16.7) |
0 |
0 |
1 (8.3) |
0 |
1 (16.7) |
0 |
0 |
0 |
2 (28.6) |
0 |
|
Nausea |
0 |
3 (50.0) |
1 (16.7) |
0 |
0 |
0 |
0 |
1 (16.7) |
2 (33.3) |
1 (16.7) |
1 (16.7) |
1 (16.7) |
2 (28.6) |
3 (23.1) |
|
Vomiting |
0 |
1 (16.7) |
0 |
0 |
1 (16.7) |
0 |
1 (8.3) |
1 (16.7) |
2 (33.3) |
0 |
0 |
0 |
1 (14.3) |
0 |
To cite this abstract in AMA style:
Lu P, Fleischmann R, Curtis C, Ignatenko S, Desai M, Wong SL, Grebe KM, Zeng J, Medema J, Stolzenbach J. Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of the BCL-2 Inhibitor Venetoclax (ABT-199) in a Phase 1 Single and Multiple Ascending Dose Study in Female Patients with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/safety-tolerability-pharmacokinetics-and-pharmacodynamics-of-the-bcl-2-inhibitor-venetoclax-abt-199-in-a-phase-1-single-and-multiple-ascending-dose-study-in-female-patients-with-systemic-lupus-er/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-tolerability-pharmacokinetics-and-pharmacodynamics-of-the-bcl-2-inhibitor-venetoclax-abt-199-in-a-phase-1-single-and-multiple-ascending-dose-study-in-female-patients-with-systemic-lupus-er/