Session Information
Session Type: Late-Breaking Abstract Session
Session Time: 7:30AM-9:00AM
Background/Purpose: Over 50 years have passed since the last therapy was approved for cutaneous lupus erythematosus (CLE).1 Parenteral administration, off-label use, or toxicity with long-term use of some existing therapies illustrate a key unmet need to identify novel treatments for CLE. Toll-like receptors (TLR) are implicated in SLE pathobiology, which make these receptors candidate targets for CLE therapies.2 Afimetoran is a first-in-class, orally bioavailable, potent, and selective small molecule inhibitor of TLR7 and TLR8. This phase 1b, randomized, double-blind, placebo-controlled study (NCT04493541) investigated the safety, tolerability, and exploratory efficacy of afimetoran in patients with CLE.
Methods: Patients were aged 18–65 years, diagnosed with either SLE according to EULAR/ACR 2019 classification criteria3 or had biopsy-proven CLE, had a modified CLE Disease Area and Severity Index-Activity (CLASI-A) score of ≥ 6, were antinuclear antibody positive, and had no evidence of retinal toxicity. Stable doses of oral corticosteroids and/or antimalarials at baseline were permitted. Patients were randomized 2:1 to once-daily oral afimetoran (30 mg) or placebo for 16 weeks and monitored for 4 weeks after stopping treatment. The primary endpoints were safety and tolerability; efficacy was an exploratory endpoint.
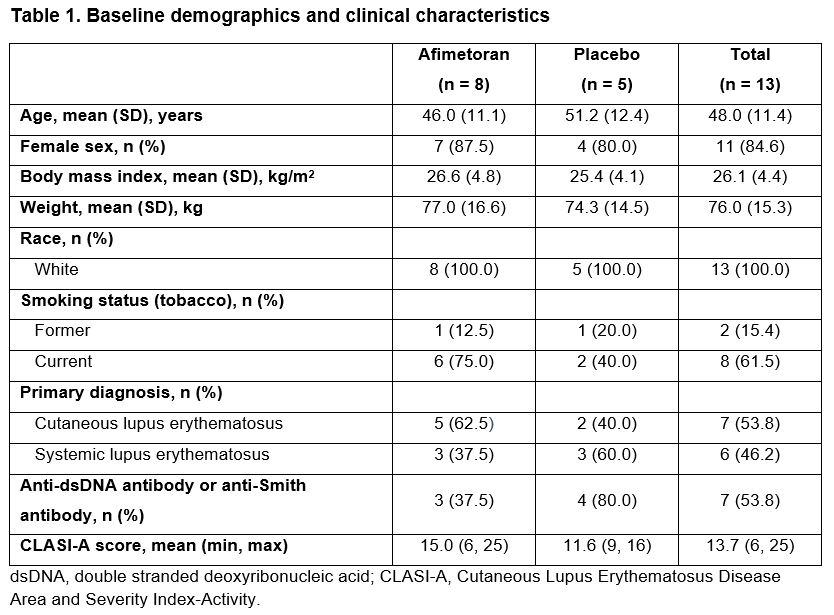
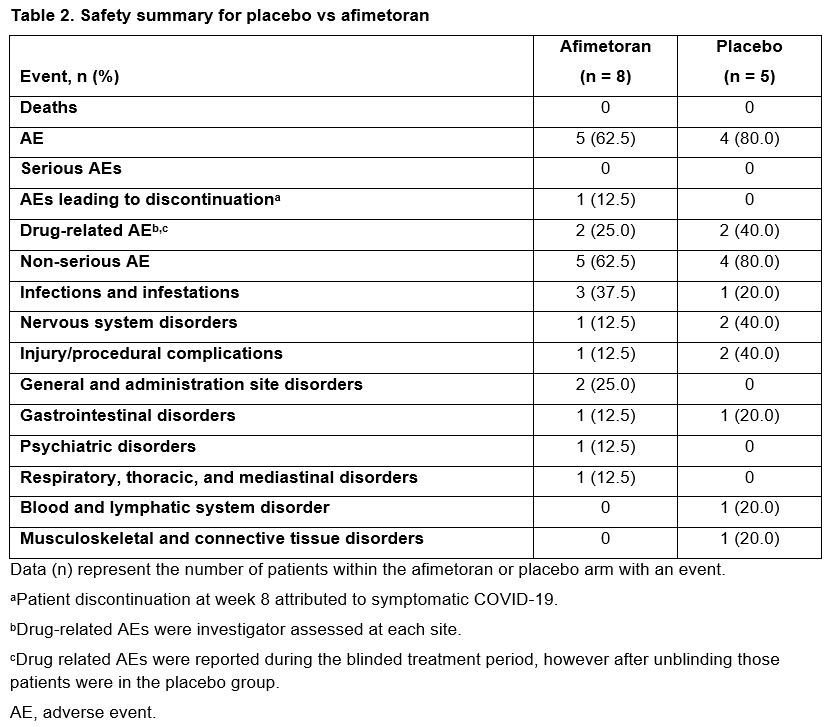
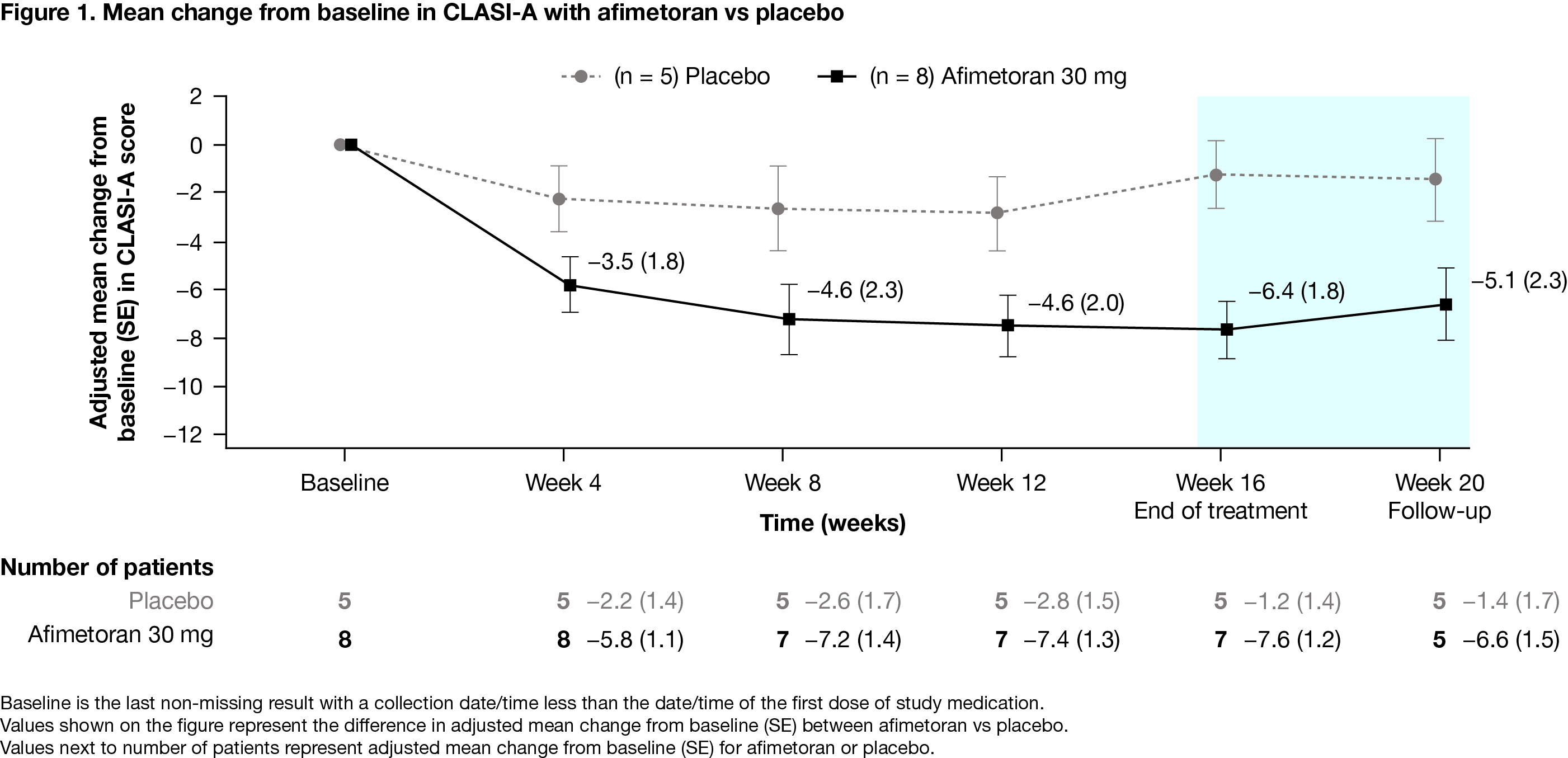
Results: 13 patients were randomized (afimetoran, n = 8; placebo, n = 5); 12 patients completed 16 weeks of treatment and 1 discontinued from the afimetoran arm due to an adverse event (AE) of symptomatic COVID-19 infection. Baseline patient characteristics were generally balanced, though the patients in the afimetoran arm had higher baseline CLASI-A scores and a higher proportion of current smokers vs placebo (Table 1). The primary objective was met: afimetoran demonstrated a favorable safety profile and was well tolerated vs placebo. Further, there were no serious AEs or safety signals among laboratory tests, vital signs, or electrocardiography. Overall, AEs were mild or moderate and resolved by the end of the study without intervention (Table 2). The proportion of patients with reported AEs was lower for patients treated with afimetoran vs placebo (62.5% vs 80.0%, Table 2). Compared with placebo, patients treated with afimetoran showed a greater reduction in CLASI-A scores as early as week 4 (first CLASI-A assessment point) which continued through the 16 weeks of active treatment and persisted for 4 weeks after end of treatment (Figure 1).
Conclusion: Afimetoran demonstrated a favorable safety profile and was well tolerated vs placebo in patients with CLE. These results provide the first clinical evidence to suggest that afimetoran may offer a clinical benefit as indicated by an early and sustained clinical response vs placebo as measured by CLASI-A. As such, these results support the continued clinical investigation of afimetoran in lupus, including the ongoing phase 2b study in SLE (NCT04895696).
References:
1. Guo LN, et al. Lupus Sci Med 2021;8:e000529.
2. Ishizaka ST, et al. Eur J Pharmacol 2023;957:175962.
3. Aringer M, et al. Arthritis Rheumatol 2019;71:1400–1412
Medical writing: Mohammad Tauseef (Caudex), funded by Bristol Myers Squibb.
To cite this abstract in AMA style:
Hosein F, Ignatenko S, Chadwick K, Zhu L, Baribaud F, Bach T, Karabeber H, Dawes M, Carayannopoulos L, Krishna G. Safety, Tolerability, and Exploratory Efficacy of Afimetoran, a TLR7/8 Inhibitor, in Patients with Cutaneous Lupus Erythematosus: A Phase 1b Randomized, Double-Blind, Placebo-Controlled Study [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/safety-tolerability-and-exploratory-efficacy-of-afimetoran-a-tlr7-8-inhibitor-in-patients-with-cutaneous-lupus-erythematosus-a-phase-1b-randomized-double-blind-placebo-controlled-study/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-tolerability-and-exploratory-efficacy-of-afimetoran-a-tlr7-8-inhibitor-in-patients-with-cutaneous-lupus-erythematosus-a-phase-1b-randomized-double-blind-placebo-controlled-study/