Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Ixekizumab (IXE) was shown to be superior to adalimumab (ADA) in achievement of simultaneous improvement of joint and skin disease (American college of Rheumatology [ACR]50 and Psoriasis Area and Severity Index [PASI]100) in patients with active psoriatic arthritis (PsA) and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs).1 The objective of this study was to compare the safety and tolerability profile of vs ADA in patients with PsA up to 52 weeks of treatment.
Methods: SPIRIT-H2H (NCT03151551) was an open-label, head-to-head, blinded assessor clinical trial which included patients with active PsA (≥3 tender joint count + ≥3 swollen joint count) and plaque psoriasis (body surface area ≥3%) who were inadequate responders to csDMARD therapy but naïve to biologic DMARDs. Patients were randomized (1:1) to approved dosing of IXE or ADA. Safety events were assessed at each patient visit up to Week 52. Frequencies of adverse events (AEs) were based on the number of patients in the safety population (patients who received ≥1 dose of study drug). Cases of inflammatory bowel disease (IBD) and cerebro-cardiovascular events were adjudicated by external committees. Kaplan-Meier analysis of time to onset of serious adverse events (SAEs) was performed.
Results: Of the 283 patients randomized to each treatment, 87% (246/283) of patients who received IXE and 84% (237/283) of patients who received ADA, completed 52 weeks of treatment. The frequency of treatment-emergent AEs (TEAEs) was similar between the groups (74% IXE vs 69% ADA), however fewer severe TEAEs were reported in the IXE group (3.2% IXE vs 7.1% ADA) (Table). SAEs were significantly more frequent in the ADA group compared to the IXE group (12% vs 4.2%; p< 0.001), and the time to develop a patient’s first SAE was significantly shorter for ADA versus IXE (p< 0.001; Figure). Discontinuations due to AEs were numerically more frequent in the ADA group versus the IXE group (7.4% vs 4.2%; p=0.15). IXE-treated patients reported more injection-site reactions (ISR) than ADA-treated patients (11% vs. 3.5%; p=0.002). Study withdrawals due to ISR were comparable, and only one injection-site reaction was severe on ADA (Table). There were two IBD cases reported for IXE; one case was confirmed as IBD.
Conclusion: Safety results were consistent with previous trials with IXE and ADA. Compared with IXE, patients with PsA treated with ADA had significantly more serious AEs.
Reference:
1. Mease PJ, et al. Ann Rheum Dis. 2020;79(1):123-31.
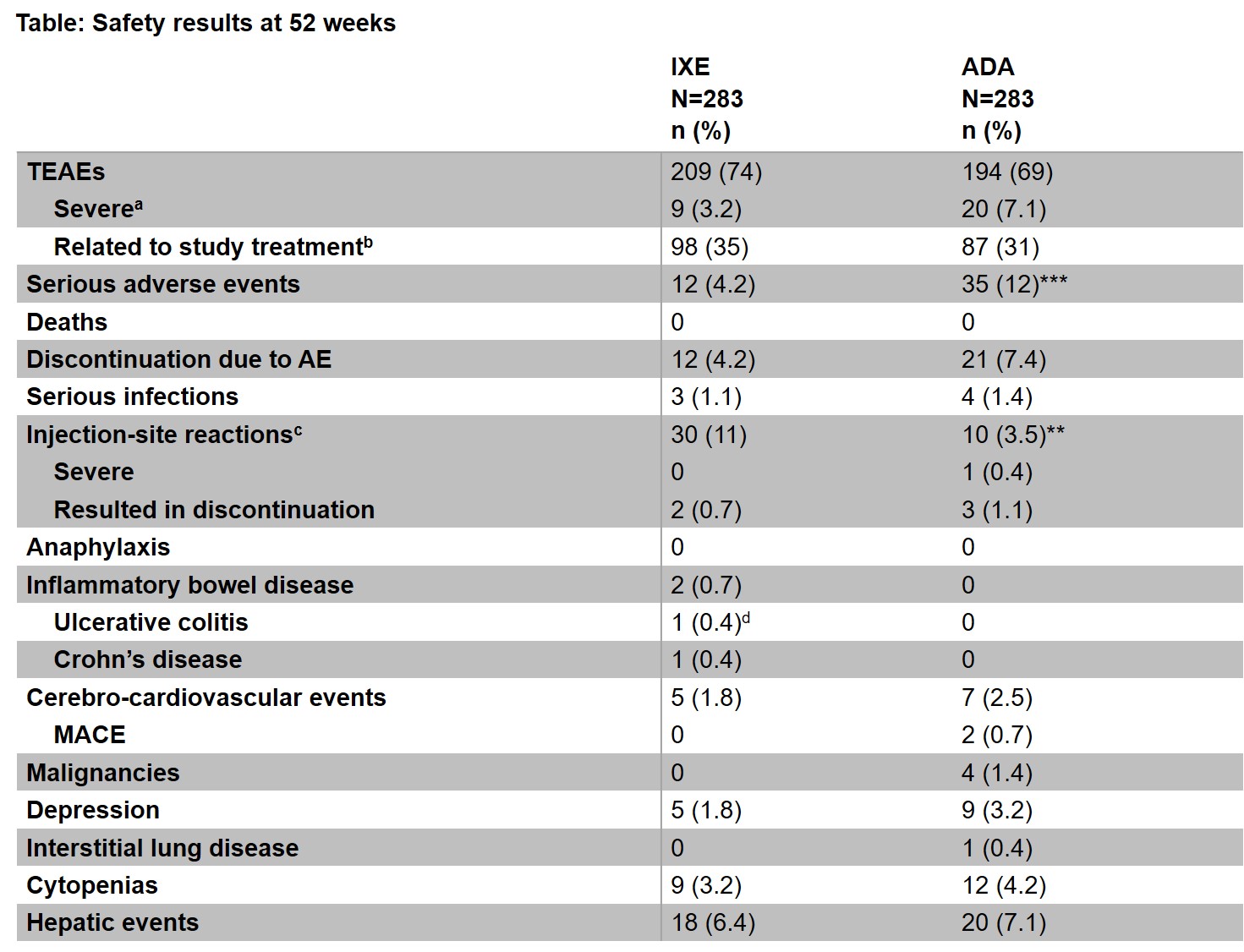 ᵃPatients with multiple occurrences of the same event are counted under the highest severity. ᵇThe treatment-emergent adverse event ’s relationship to study treatment was judged by the investigator. ᶜMedical Dictionary for Regulatory Activities (MedDRA) high-level term. ᵈThis event was adjudicated but it was not a confirmed inflammatory bowel disease. ***p < 0.001; **p < 0.01 by Fisher’s exact test. ADA=adalimumab, AE=adverse event; IBD=inflammatory bowel disease; IXE=ixekizumab; MACE=major adverse cardiovascular event.
ᵃPatients with multiple occurrences of the same event are counted under the highest severity. ᵇThe treatment-emergent adverse event ’s relationship to study treatment was judged by the investigator. ᶜMedical Dictionary for Regulatory Activities (MedDRA) high-level term. ᵈThis event was adjudicated but it was not a confirmed inflammatory bowel disease. ***p < 0.001; **p < 0.01 by Fisher’s exact test. ADA=adalimumab, AE=adverse event; IBD=inflammatory bowel disease; IXE=ixekizumab; MACE=major adverse cardiovascular event.
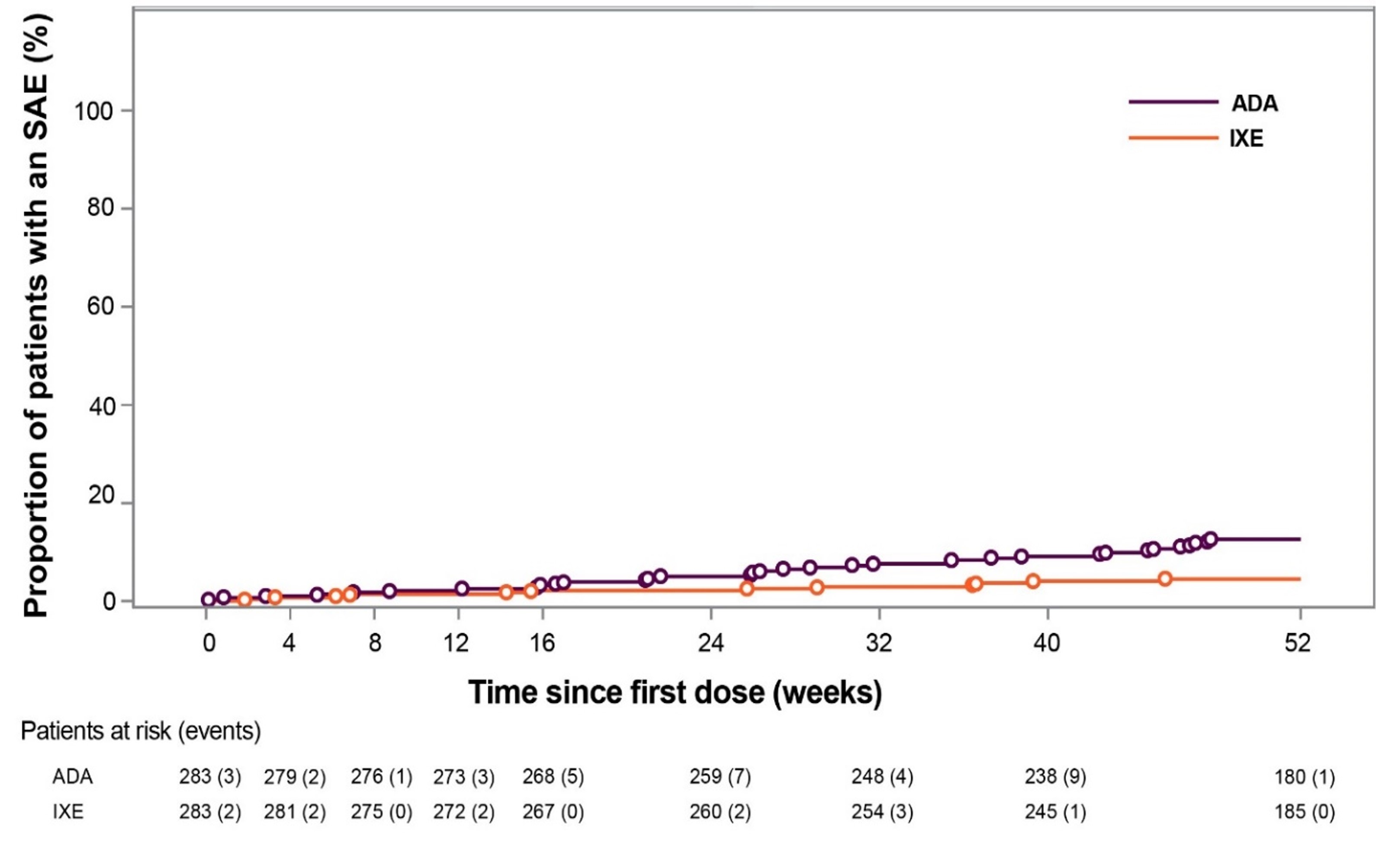 Figure: Time to onset of first SAE. Numbers below x-axis represent patients at risk at each time point. Open circles represent events. p < 0.001 by log rank test. ADA=adalimumab; IXE=ixekizumab; SAE=serious adverse event.
Figure: Time to onset of first SAE. Numbers below x-axis represent patients at risk at each time point. Open circles represent events. p < 0.001 by log rank test. ADA=adalimumab; IXE=ixekizumab; SAE=serious adverse event.
To cite this abstract in AMA style:
Mease P, Smolen J, Kavanaugh A, Nash P, Gallo G, Liu-Leage S, Sapin C, Genovese M. Safety Profiles of Ixekizumab versus Adalimumab: 52-Week Results from a Head-to-Head Comparison in Patients with Active Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/safety-profiles-of-ixekizumab-versus-adalimumab-52-week-results-from-a-head-to-head-comparison-in-patients-with-active-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-profiles-of-ixekizumab-versus-adalimumab-52-week-results-from-a-head-to-head-comparison-in-patients-with-active-psoriatic-arthritis/
