Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Atacicept targets the B-cell stimulating factors, BLyS and APRIL, and has demonstrated a clinical response in SLE patients (pts) with high disease activity (HDA; SLEDAI-2K ≥10) at Screening in the Phase IIb ADDRESS II study.
Methods: In ADDRESS II (NCT01972568), pts received weekly atacicept (75 or 150 mg SC injection) or placebo (PBO) for 24 weeks (wks) (1:1:1 randomization). Those who completed treatment were eligible to enter a long-term extension (LTE), to either continue on the same atacicept dose (atacicept groups), or switch from PBO to atacicept 150 mg (PBO/150 mg). The LTE primary objective was to determine the long-term safety and tolerability of atacicept in SLE pts. Current cumulative safety results from ADDRESS II and the LTE are reported here.
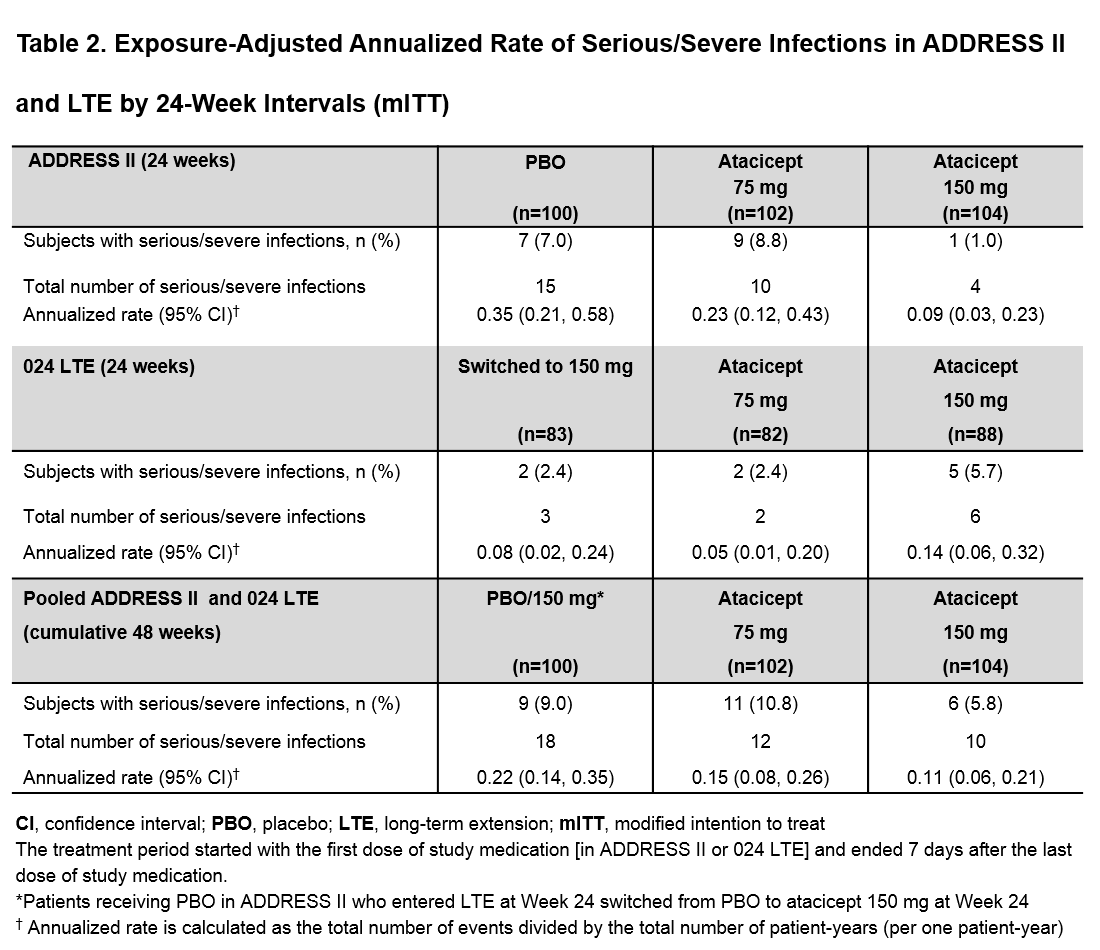
Results: Of the 306 pts in ADDRESS II, 253 (95% of completers) entered the LTE, of which 132 (52%) had HDA. By 48 wks (from start of Day 1 in ADDRESS II), most pts had ≥1 treatment-emergent adverse event (TEAE), with a slight increase in the atacicept groups vs PBO/150 mg. Herpes zoster incidence was low. Only one patient with asthma and allergic rhinitis on chronic inhaled corticosteroids developed a non-serious opportunistic infection (candida esophagitis, grade 1), which resolved with oral anti-fungal treatment. The most frequent serious TEAEs were infections (Table 1). There was no increase in the atacicept groups vs PBO/150 mg in the incidence of serious infections or exposure-adjusted annualized rates of serious/severe infections in the modified intention to treat population (Table 2) or the HDA subpopulation (data not shown). Serious/severe infections in the PBO/150 mg group occurred more frequently during the PBO-treatment period (15/18 [83%]). Serum IgG reductions (including IgG <3 g/L) were not associated with infections. Four pts in the atacicept 150 mg group developed IgG < 3 g/L after 36 wks of treatment, which resolved without treatment discontinuation; 1 patient in the atacicept 75 mg group developed IgG <3 g/L after treatment discontinuation due to lupus nephritis flare. No safety new signals were identified. The two deaths reported in the LTE occurred after 48 wks; overall exposure-adjusted mortality rate of atacicept treatment was 0.57 /100 pt-years.
Conclusion: Cumulative data demonstrate an acceptable safety profile for atacicept, with no increased risk of serious/severe infections, or mortality. This was also seen in the HDA subpopulation, in which a greater atacicept treatment effect has been observed. IgG decreases were not associated with infections but should continue to be monitored.
To cite this abstract in AMA style:
Merrill JT, Wallace DJ, Vazquez-Mateo C, Kao AH, Fleuranceau-Morel P, Chang P, Isenberg DA. Safety Profile in SLE Patients Treated with Atacicept in a Phase IIb Study (ADDRESS II) and Its Extension Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/safety-profile-in-sle-patients-treated-with-atacicept-in-a-phase-iib-study-address-ii-and-its-extension-study/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-profile-in-sle-patients-treated-with-atacicept-in-a-phase-iib-study-address-ii-and-its-extension-study/