Session Information
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Patients treated with biologic or targeted synthetic (b/ts) DMARDs are recommended to perform safety blood monitoring every 3 months in the absence of strong evidence for this recommendation, especially related to TNF inhibitors (TNFi).
This study aimed to assess the need for routine safety blood monitoring during treatment with b/tsDMARDs.
Methods: A national health Organization (insuring ~50% of the population) database retrospective cohort study. Adult patients diagnosed with RA, SpA, PsA, and IBD treated with a b/tsDMARD as monotherapy with a follow-up of ≥12 months after the start of medication were included. Demographic and clinical data were retrieved from the electronic medical files.
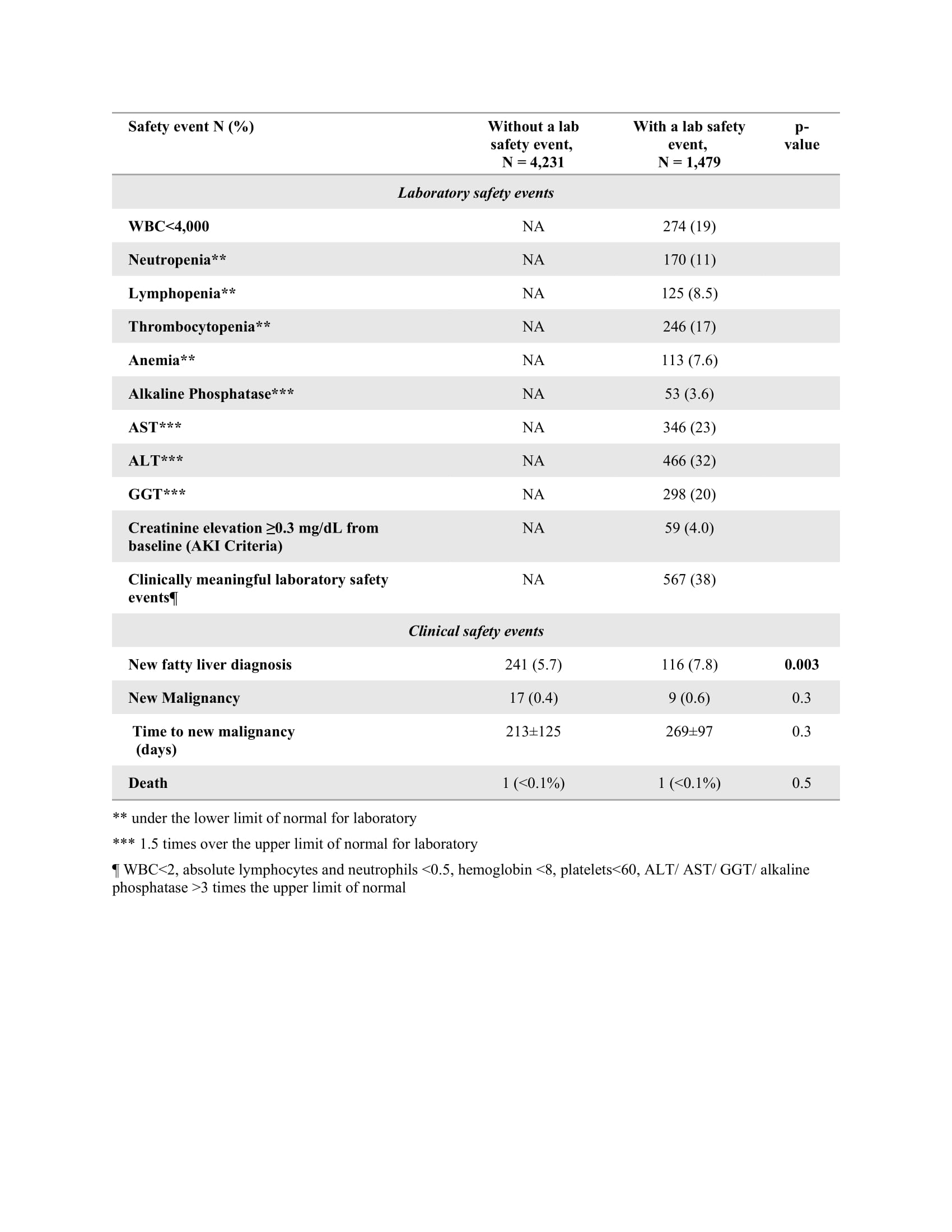
A safety lab event included cytopenia, abnormal liver function tests (LFT), and kidney injury. A safety clinical event included a new diagnosis of fatty liver, malignancy, or death.
The primary outcome was the rate of clinical safety events in patients with/without a lab event. Secondary objectives were rate of adherence to testing every 3 months (per-protocol), and effect of DMARD mode of action, rheumatologic diagnosis, and comorbidity on drug-related adverse events.
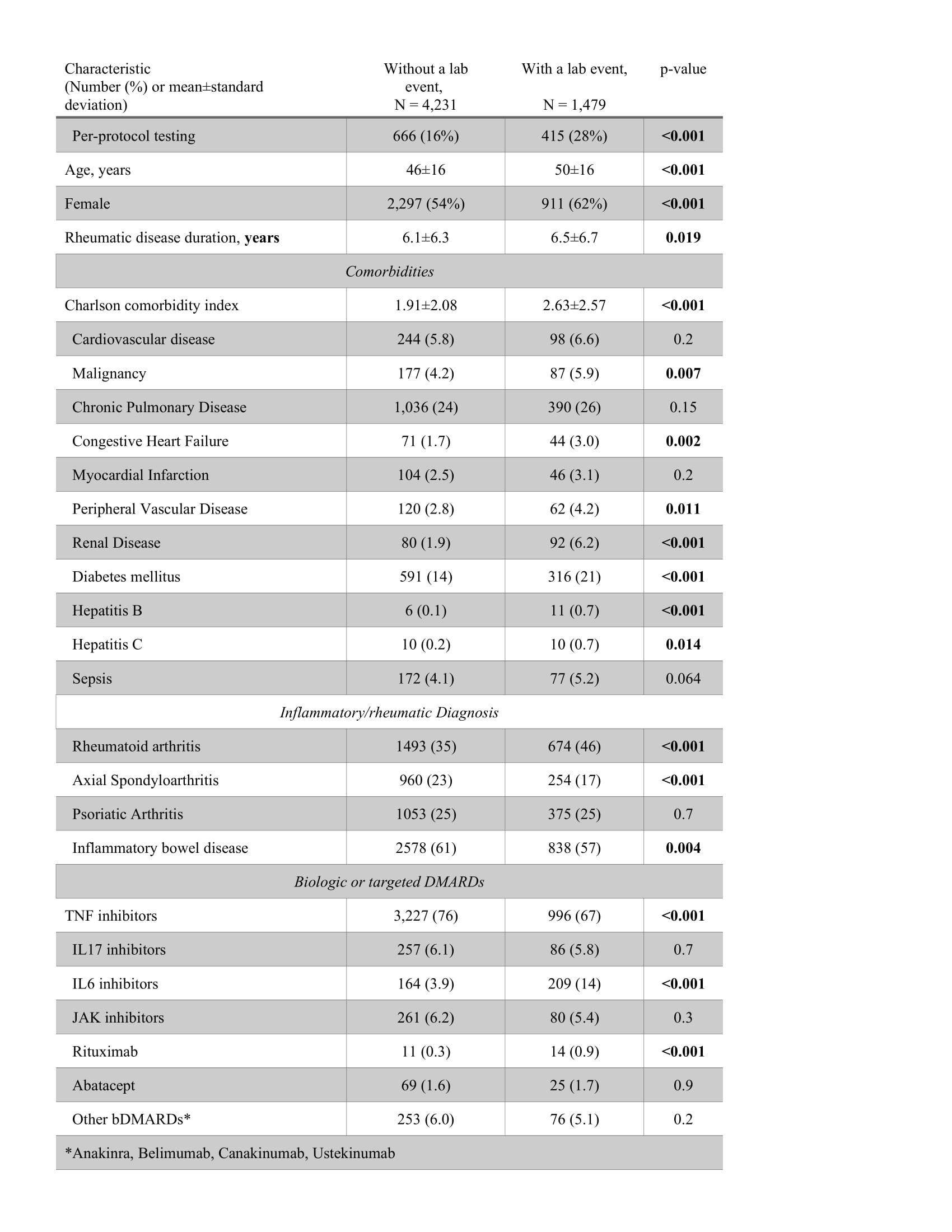
Results: A total of 5710 cases were included, of them 1081 (18.9%) adhered to per-protocol testing, and 4629 (81.1%) did not. A lab safety event was recorded for 1479 (25.9%) cases, of them 38% were considered clinically meaningful, with elevation of LFT being the most common (Table 1). Cases who had a lab event were more likely to adhere to per-protocol testing (28 vs 16%), be older (50±16 vs 46±16 years), and have a higher Charlson comorbidity index score (2.6±2.6 vs 1.9±2.1); they were more likely to be diagnosed with RA (41 vs 31%), and less likely to have SpA (17 vs 23%), less likely to be treated with a TNFi (67 vs 76%) and more likely to be treated with an IL-6i (14 vs. 3.9%) or rituximab (0.9 vs. 0.3%), p≤0.001 for all comparisons (Table 2).
Participants with a lab event had similar rates of new malignancy (0.6 vs 0.4%, p=0.3) and death ( >0.1% vs >0.1%, p=0.5), but higher rates of fatty liver 7.8 vs 5.7% (p=0.003) (Table 1).
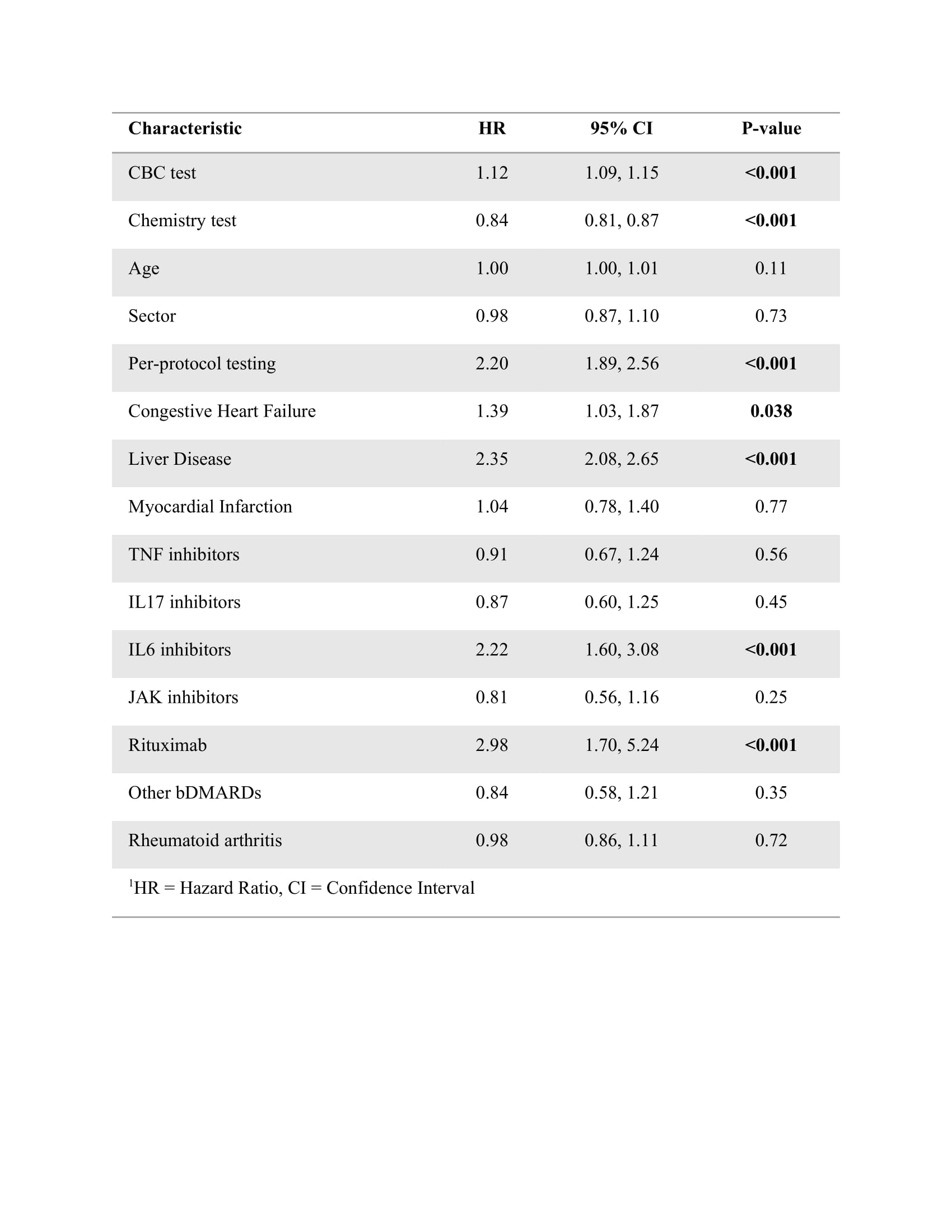
In a Cox regression analysis multivariable model, being adherent to per-protocol testing (hazard ratio (HR) (95%CI) 2.2 (1.89-2.56)), CHF (1.39 (1.03-1.87), liver disease (2.35 (2.08-2.65) and being treated with IL6i (2.22 (1.6-3.08) and rituximab (2.98 (1.7-5.24) were associated with an increased risk for all (clinical and lab) safety events (Table 3). Results were similar in a model for lab events only. In a model for clinical events, only age (1.02 (1.01-1.03), CHF (2.29 (1.41-3.7), and liver disease (2.79 (2.26-3.43) were associated with an increased HR.
Conclusion: Only a fifth of patients treated with b/tsDMARDs adhere to the recommended routine blood tests every 3 months. Per-protocol testing expectedly revealed more lab abnormalities, but lab events were not associated with an increased HR for new malignancy or death. Comorbidities and being treated with IL6i and rituximab were associated with an increased risk for all events, while TNFi and RA did not.
There may be a rationale for differential recommendations regarding routine blood testing depending on DMARD used and comorbidities, which may decrease health care utilization and financial costs.
To cite this abstract in AMA style:
Eviatar T, Sagy I, Brav E, Bieber A, Furer V, Elkayam O. Safety Outcomes of Routine Blood Tests for Monitoring of Biologic and Targeted Synthetic Therapies in Inflammatory Arthritis Patients [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safety-outcomes-of-routine-blood-tests-for-monitoring-of-biologic-and-targeted-synthetic-therapies-in-inflammatory-arthritis-patients/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-outcomes-of-routine-blood-tests-for-monitoring-of-biologic-and-targeted-synthetic-therapies-in-inflammatory-arthritis-patients/