Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: Secukinumab, a fully human anti-interleukin (IL)-17A monoclonal antibody, is approved for multiple immunological disorders, including moderate-to-severe plaque psoriasis (PsO), psoriatic arthritis (PsA), axial spondyloarthritis (radiographic and non-radiographic axSpA), and hidradenitis suppurativa (HS). Long-term safety of secukinumab was previously reported based on pooled data from 47 clinical trials involving 15,644 patients with PsO, PsA and axSpA, with a cutoff until June 25, 2022.1 Herein, we report the updated safety profile of secukinumab treated adult patients based on a larger pool of clinical trials and including a newly approved indication of moderate to severe HS.
Methods: The pooled safety analysis included data from 69 clinical trials (PsO: 41; PsA: 13; axSpA [including radiographic and non-radiographic axSpA]: 13 trials; HS: 2 trials) of patients who had received ≥1 dose of secukinumab (cutoff date: December 2023). Adverse events (AEs) were reported as exposure-adjusted incidence rates (EAIRs)/100 PYs.
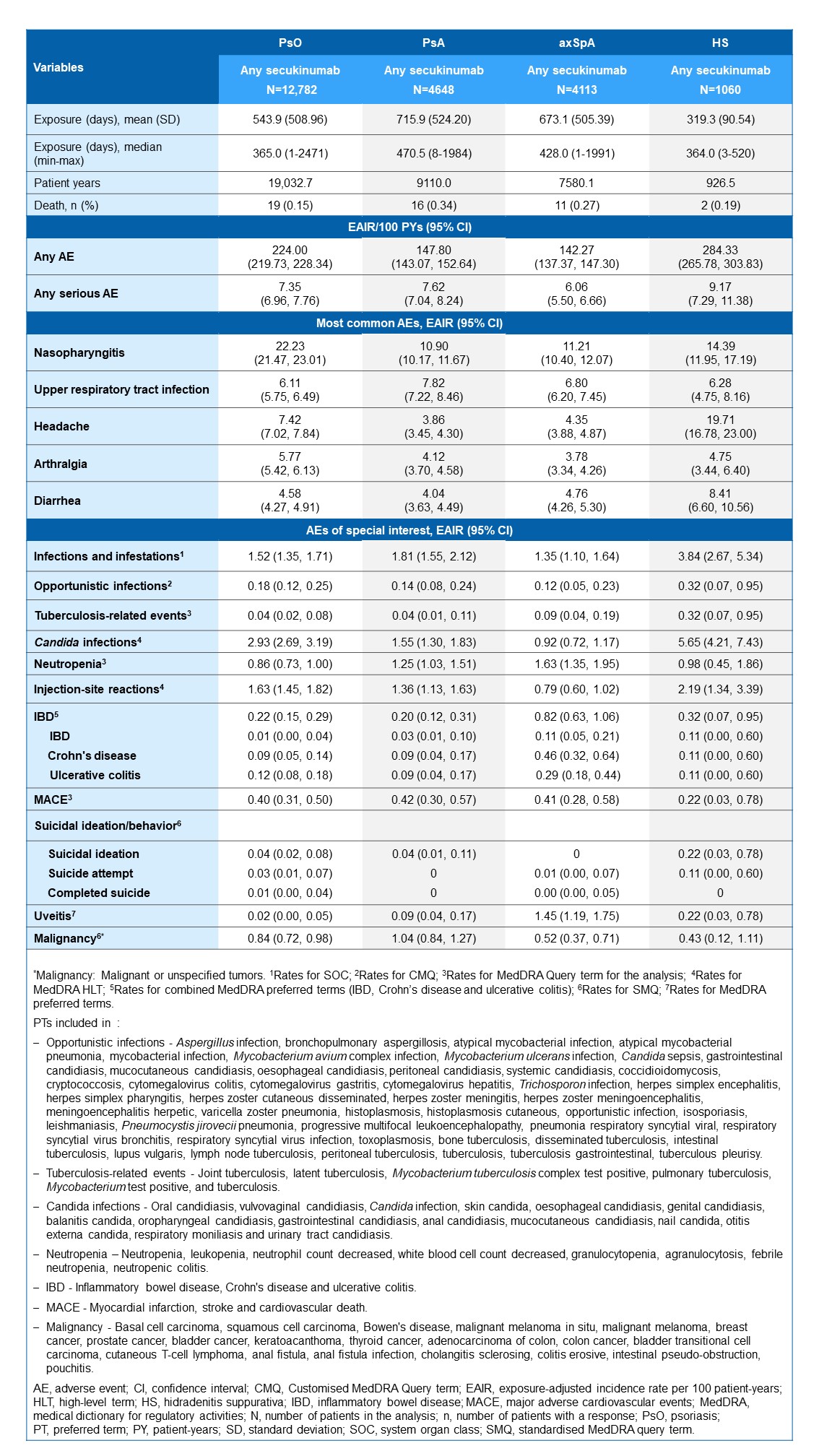
Results: A total of 22,603 patients (PsO [N=12,782], PsA [N=4648], axSpA [N=4113], HS [N=1060]) with overall exposure of 36,649.3 PYs were included in the analysis. The most frequent AEs reported were nasopharyngitis, upper respiratory tract infection and headache (Table 1). EAIRs per 100 PYs for inflammatory bowel disease, malignancies, major adverse cardiovascular events, and suicidal ideation remained low across all indications (Table 1).
Conclusion: This pooled safety data analysis, including long-term exposure data from 69 clinical trials including 22,603 patients, reaffirms the consistent safety profile of secukinumab in a large pool of clinical trials in patients with PsO, PsA, axSpA, and moderate to severe HS. Adequate tolerability was observed in patients exposed up to a mean of 470.5 days, consistent with the safety profile characterized during the placebo-controlled period across indications.
Reference
Sun R, et al. Dermatol Ther (Heidelb). 2024;14(3):729-743.
To cite this abstract in AMA style:
Deodhar A, McInnes I, Baraliakos X, Gottlieb A, Kiltz U, Schreiber S, Sahoo B, Bao W, Gaillez C, Bustamante M, Mease P. Safety of Secukinumab in Patients with Psoriasis, Psoriatic Arthritis, Axial Spondyloarthritis and Hidradenitis Suppurativa: Updated Pooled Data from 69 Clinical Trials [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safety-of-secukinumab-in-patients-with-psoriasis-psoriatic-arthritis-axial-spondyloarthritis-and-hidradenitis-suppurativa-updated-pooled-data-from-69-clinical-trials/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-secukinumab-in-patients-with-psoriasis-psoriatic-arthritis-axial-spondyloarthritis-and-hidradenitis-suppurativa-updated-pooled-data-from-69-clinical-trials/