Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Low-dose trimethoprim-sulfamethoxazole (LDTS, 160mg-800mg 3x/week or 80mg-400mg/day) is effective for prevention of Pneumocystis jiroveci pneumonia (PCP), a serious opportunistic infection seen in patients with ANCA-associated vasculitis (AAV) receiving immunosuppression. There have been rare reports of profound cytopenias when therapeutic-dose TS (160mg-800mg 2x/day) was added to methotrexate (MTX), raising concern about whether LDTS could be given for PCP prophylaxis in AAV patients receiving MTX. This study sought to examine the safety of MTX+LDTS in a well characterized cohort of patients with AAV.
Methods:
A retrospective chart review was performed of AAV patients treated with MTX at our Center 2002-2017. Clinical variables and laboratory data were collected in RedCap. Kaplan-Meier curves were constructed of drug discontinuation by group with differences compared using the Log-rank test and Wilcoxon test. Side effects were compared using chi-square, Fisher’s exact test, or ANOVA.
Results:
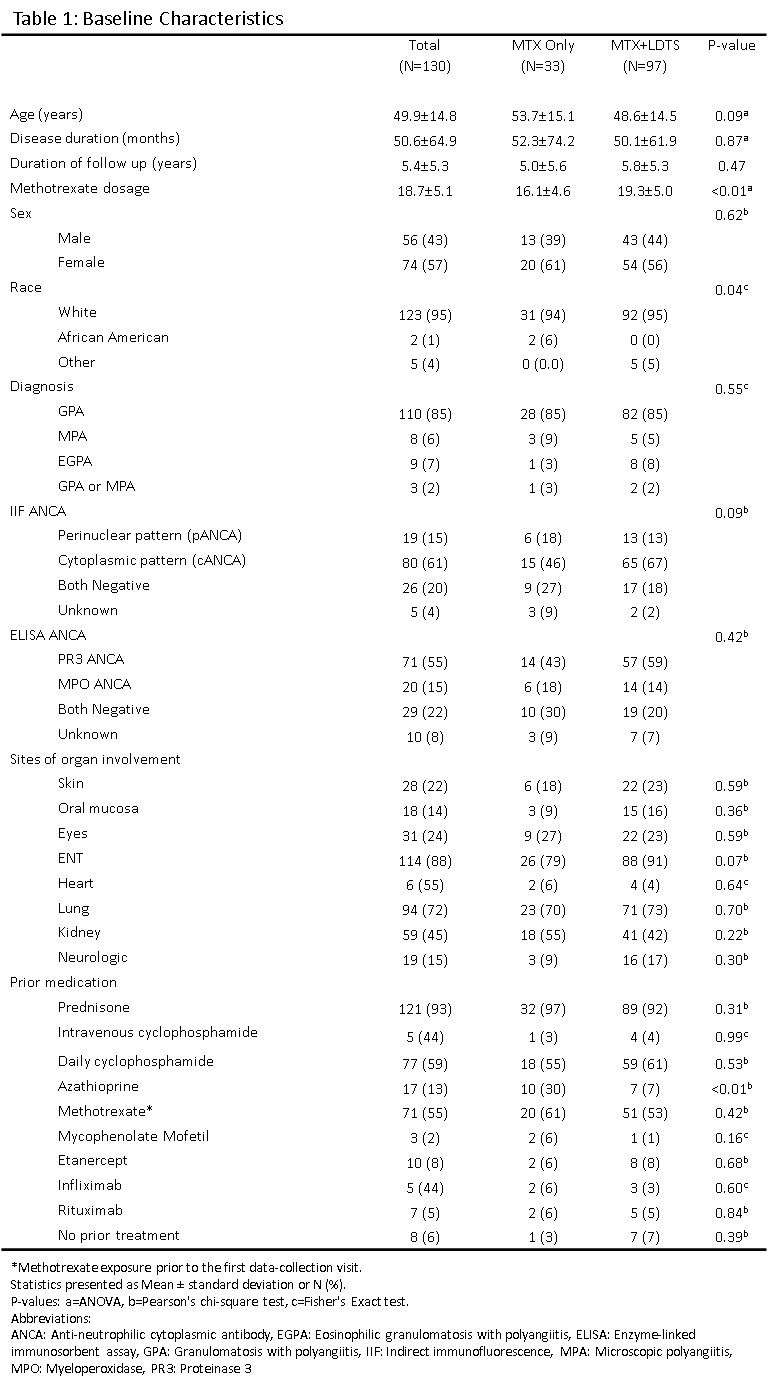
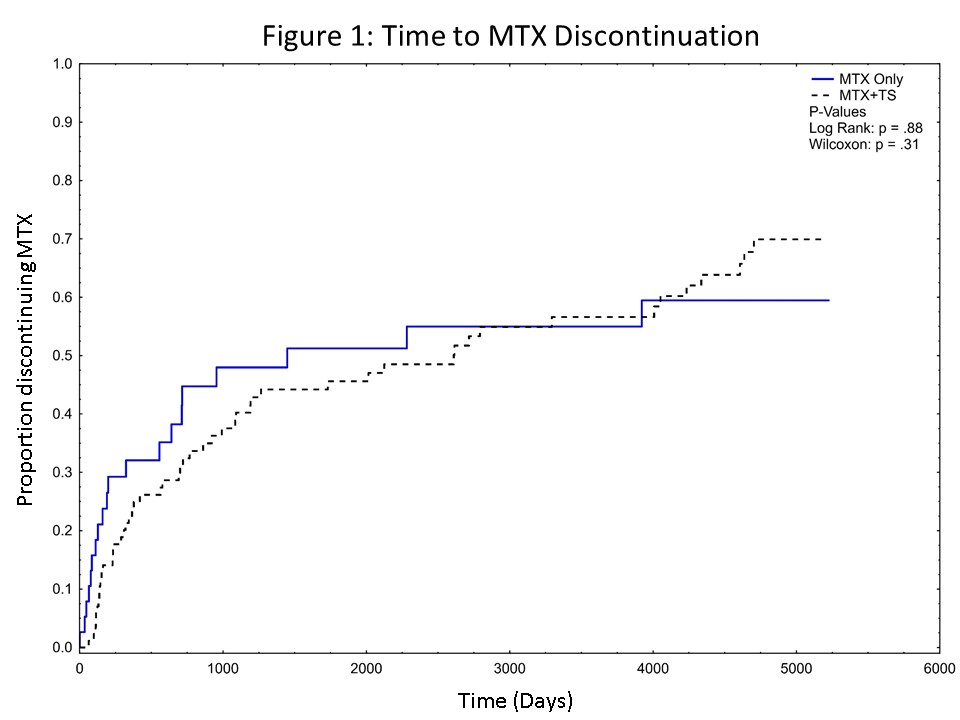
130 patients were included: MTX only (N=33), MTX+LDTS (N=97). Demographics are summarized in Table 1. The rate of discontinuation for MTX was similar between the two groups (Figure 1), with no differences seen in the 5 identified reasons for discontinuation: side effects, infection, switching to another medication for active disease, MTX withdrawal after sustained remission, and patient discontinuation. The overall occurrence of side effects was similar (8.6% [MTX only] vs 7.1% [MTX+LDTS], P=0.32). Thrombocytopenia (3.8% [MTX only] vs 1.1% [MTX+LDTS], P< 0.001) and gastrointestinal side effects (3.9% [MTX only] vs 1.6% [MTX+LDTS], P=0.005) occurred more frequently in those on MTX only but there was no difference in the frequency of leukopenia (0.8% [MTX only] vs 1.0% [MTX+LDTS], P=0.72), transaminitis (6.4 % [MTX only] vs 6.2 % [MTX+LDTS], P=0.88), hospitalization for suspected infection (2.5% [MTX only] vs 1.9.% [MTX+LDTS], P=0.52). One patient on MTX 25mg/week + LDTS had Grade 4 neutropenia that occurred following rituximab and moderate renal insufficiency.
Conclusion:
In our AAV cohort, the frequency of MTX discontinuation and side effects was similar in patients treated with MTX alone or combined with LDTS. As this study only examined those receiving LDTS, these observations do not extend to those receiving higher doses of TS together with MTX. These results provide support that MTX can safely be combined with LDTS for PCP prophylaxis in AAV. As serious bone marrow suppression can occur with MTX use, with or without LDTS, close laboratory monitoring remains important.
To cite this abstract in AMA style:
Tamaki H, Butler R, Langford CA. Safety of Methotrexate and Low-Dose Trimethoprim-Sulfamethoxazole in Patients with ANCA-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/safety-of-methotrexate-and-low-dose-trimethoprim-sulfamethoxazole-in-patients-with-anca-associated-vasculitis/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-methotrexate-and-low-dose-trimethoprim-sulfamethoxazole-in-patients-with-anca-associated-vasculitis/