Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Increased awareness of the importance of MTX in rheumatic disease is leading to more MTX use in patients from TB-endemic areas. Current management guidelines for rheumatic disease address TB in the context of biologics but not MTX use. We aimed to systematically review the published literature on TB rates with MTX < 30 mg per week.
Methods: We searched CINAHL, Embase, Global, MEDLINE and World of Science databases (Jan 1990 to May 2018) for terms including ‘methotrexate’ and ‘tuberculosis’. We also searched citations from review articles. Titles, abstracts or full manuscripts of the 4707 reports identified were screened independently by 2 reviewers to identify studies reporting TB in patients taking MTX. Study quality was assessed using the McGill Mixed Methods Appraisal Tool (MMAT). Data was extracted on TB incidence (new TB diagnosis vs reactivation of latent TB), and outcomes (pulmonary, dissemination, death) and safety of isoniazid, INH. Descriptive summaries are presented on studies providing outcomes in patients taking MTX < 30 mg per week.
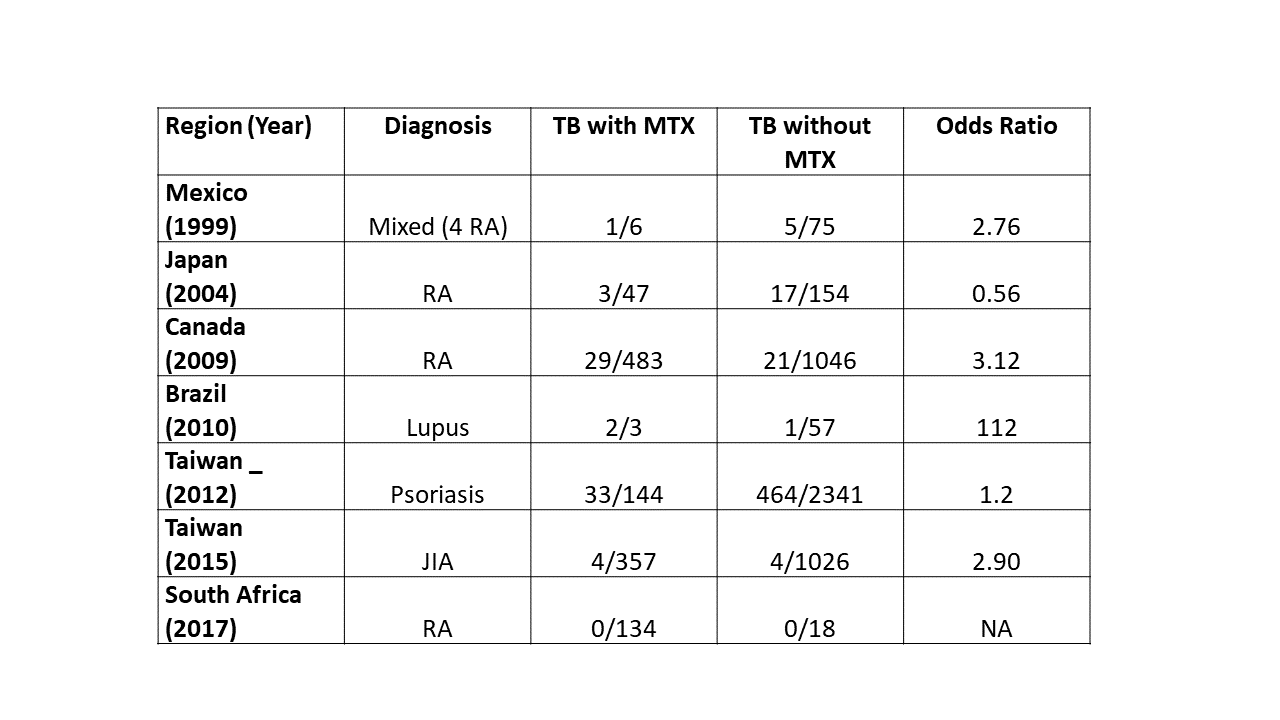
Results: After removing duplicates and studies not meeting criteria or providing sufficient information, 31 studies were included (8 cohort, 7 case-control, 1 clinical trial, 15 case reports/case series). Only 27% of articles reported data from low to moderate human development index countries. Studies were of moderate quality. Seven case control studies were heterogeneous but most demonstrated a modest increased risk of TB with MTX (Table). Five cohort studies reported TB incidence rates in rheumatic disease (treated with MTX +/- biologics) ranging from 102-367.9/100,000 patient-years. These rates were generally higher than comparator general population rates. Two cohort studies of MTX in RA (without biologic) reported cumulative TB incidence in Maldova (12 TB cases in 44 RA patients, 27%) and in China (9/114, 7.9%). Other cohort studies generated rates of overt infection (143/100,000 patient years in Spain, higher if co-prescribed with corticosteroids and other immunosuppressants in South Africa), and latent TB rates detection (16/922 RA screened, 1.7%, in Canada). When reported, rates of extra-pulmonary TB were higher than comparator general population rates. One clinical trial (China), 2 cohorts (Japan, USA) and 2 case series (Belgium, USA) evaluated safety of INH and MTX. Isoniazid-related hepatotoxicity and neutropenia were generally more common when taken with MTX, but were usually reversible.
Conclusion: Despite a paucity of high-quality data, this review confirms that TB screening and clinical surveillance are needed in patients from TB-endemic areas who are prescribed MTX, particularly with co-administration of corticosteroids or other immunosuppressants. Isoniazid, if monitored, appears safe and prevents TB reactivation.
To cite this abstract in AMA style:
Davidson A, Gunay A, Colmegna I, Lacaille D, Loewen H, Meltzer M, Tadese Y, Yirsaw Z, Bernatsky S, Hitchon C. Safety of Low Dose Methotrexate (MTX) and Tuberculosis (TB) [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/safety-of-low-dose-methotrexate-mtx-and-tuberculosis-tb/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-low-dose-methotrexate-mtx-and-tuberculosis-tb/