Session Information
Session Type: Poster Session A
Session Time: 10:30AM-12:30PM
Background/Purpose: Management of solid-organ transplant (SOT) recipients with systemic inflammatory diseases represents a clinical challenge in the paucity of data related to the safety of post-SOT biologics use. This study sought to investigate the safety outcomes in the biologic-treated kidney transplant recipients with systemic inflammatory diseases compared to SOT recipients without biologic treatment in a tertiary medical center in Israel.
Methods: A retrospective study collected data (2000-2023) on the biologic-treated adult kidney transplant recipients with systemic inflammatory diseases compared to the control counterparts without inflammatory disease not treated with biologics, matched based on age, gender, diabetes mellitus (DM), and the year (± 1) of the kidney transplantation. Safety outcomes included serious infections defined as those requiring hospitalization or leading to death, graft rejection, cancer, and death.
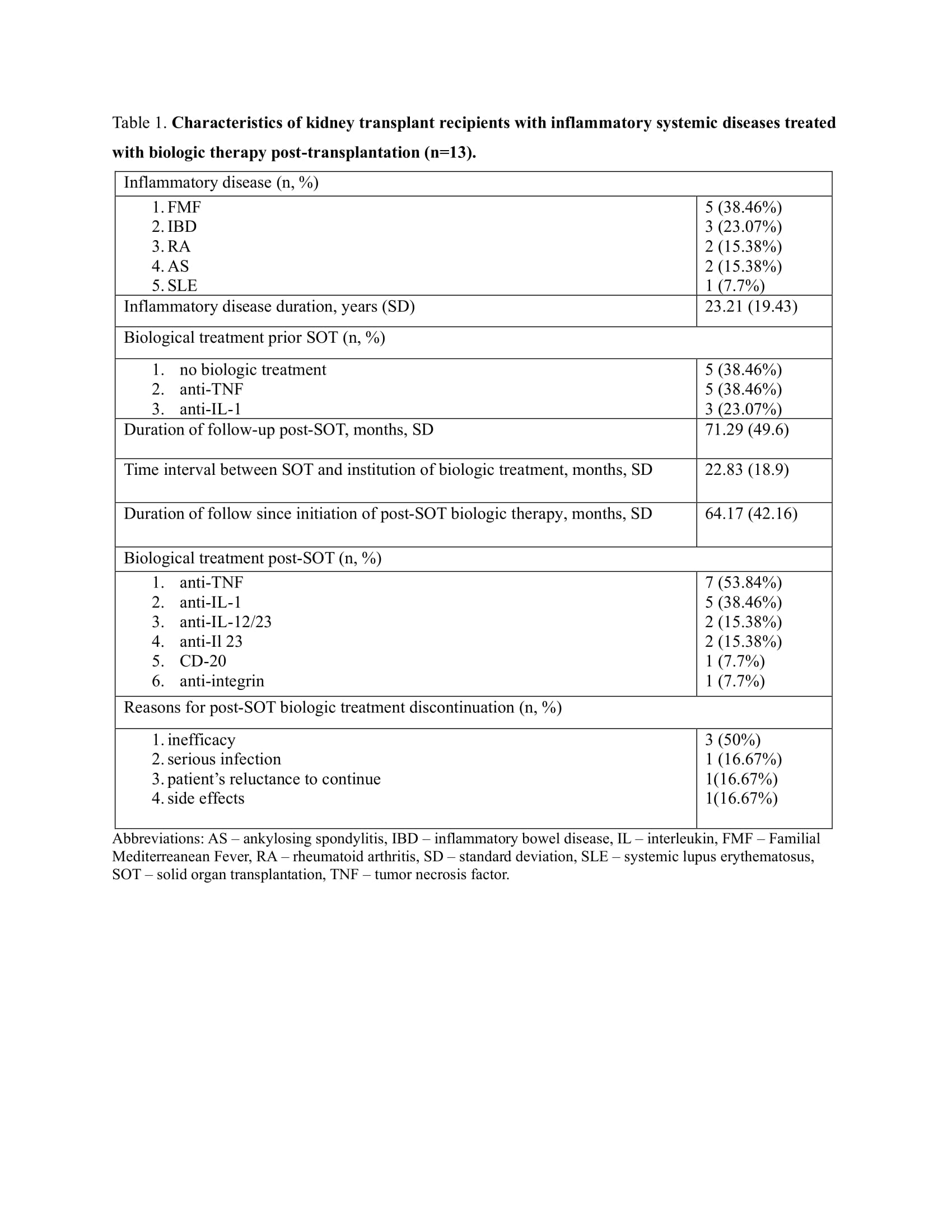
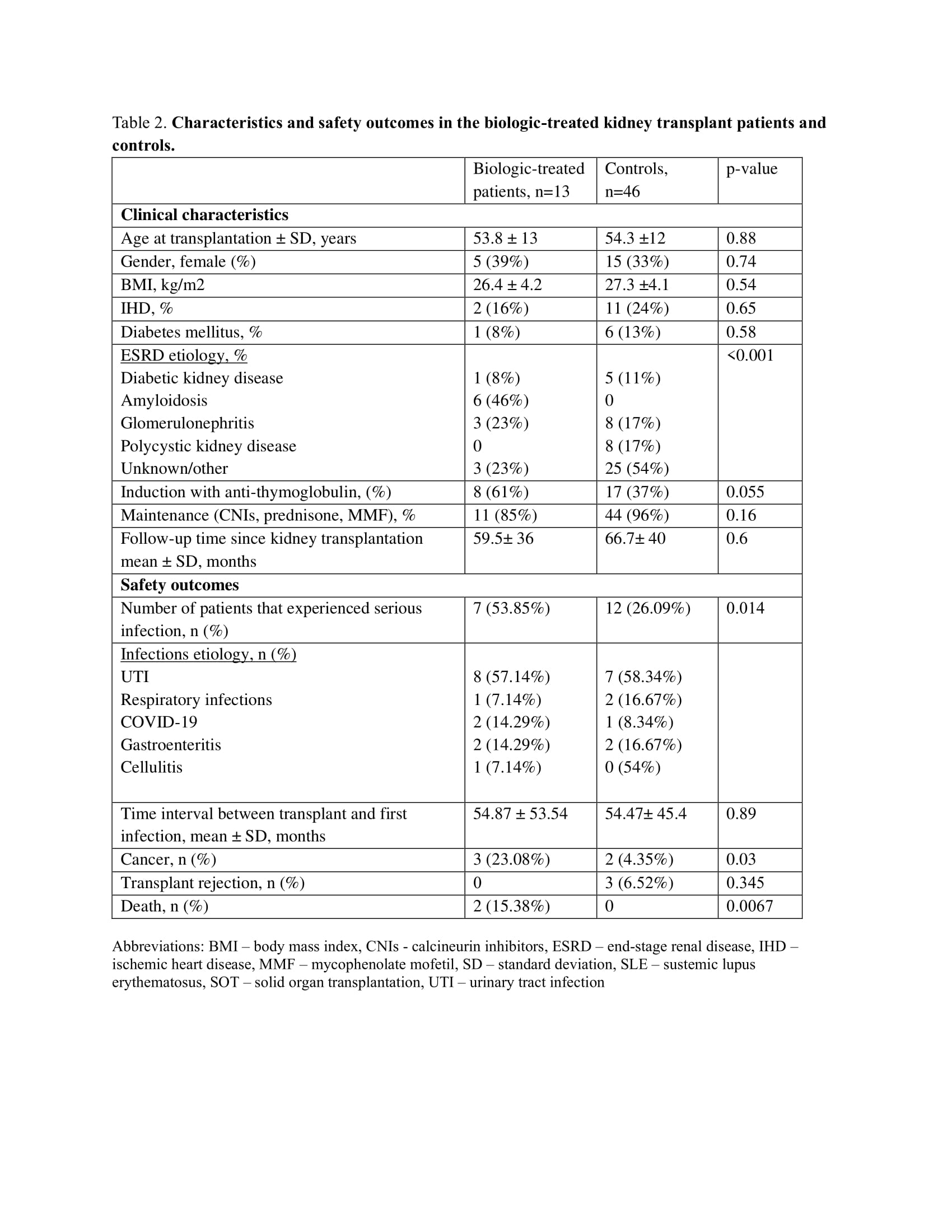
Results: A total of 13 biologic-treated (Table 1) and 46 matched living-donor kidney transplant recipient controls were included in the study (Table 2). All patients were Caucasians. The biologic-treated group predominantly consisted of males (n=8, 61%), age at transplantation (mean ± SD) 53.8 ± 13 years, with Familial Mediterranean Fever (FMF) and inflammatory bowel disease (IBD) being the most prevalent inflammatory diseases, 5 (38.46%) and 3 (23.07%), respectively. TNF and IL-1 inhibitors were the most prescribed biologics prior and post-SOT, initiated 22.83 ±18.9 months post-SOT. All patients received the same biologic prior to and post-SOT. The duration of follow-up since post-SOT biologics administration was 64.17±42.16 months.
Post-SOT serious infections were higher in the biologic-treated group, 7 (53.85%) vs 12 (26.09%), respectively. Three (23.08%) biologic-treated patients experienced multiple infections compared to none in the control group. Urinary tract infection was the prevailing infection in both groups, 8 (57.14%) and 7 (58.34%), respectively. There was no evidence of opportunistic infections in the study cohort. The time to the first infection was similar in both groups.
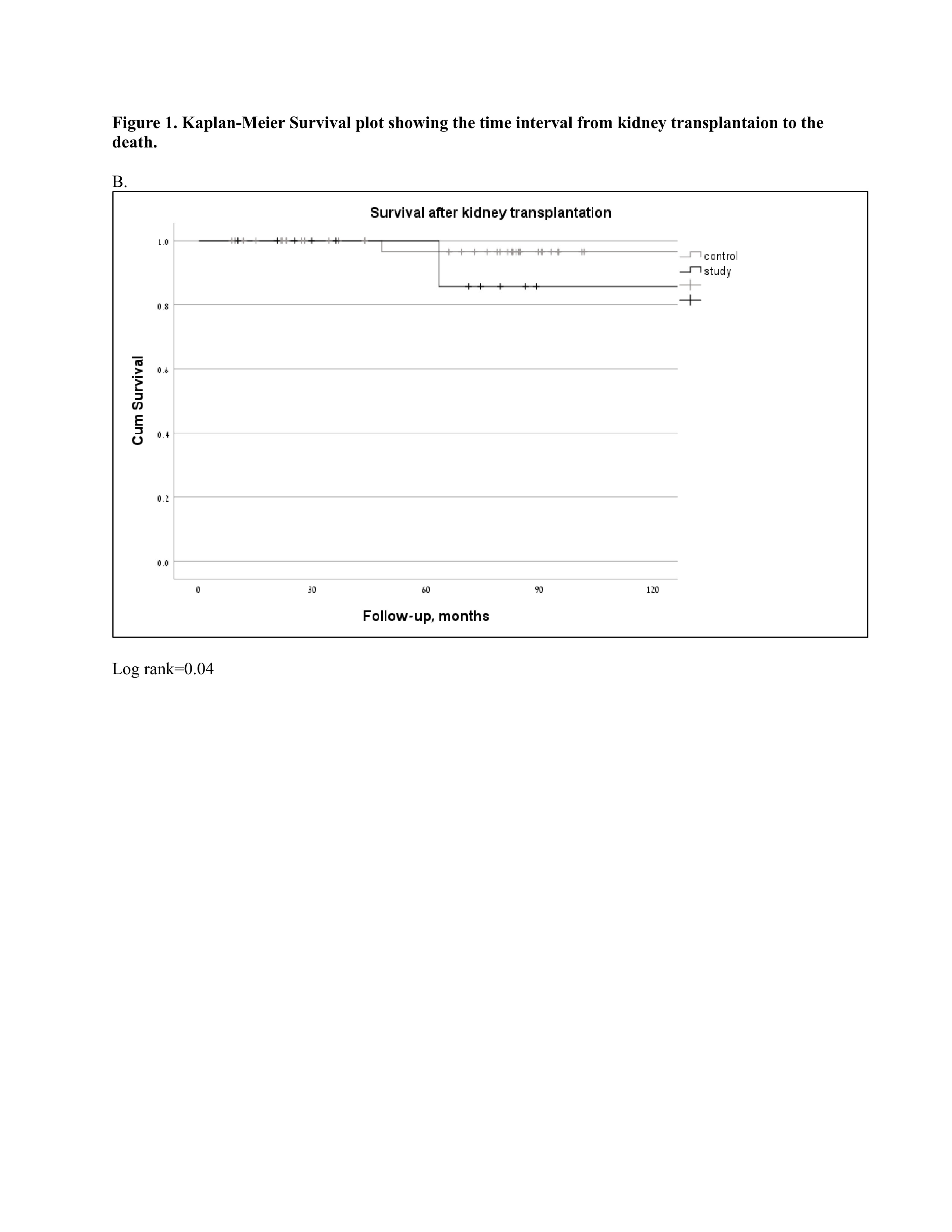
Among the biological treatment group, there were no graft rejection events, compared to 3 (6.52%) biopsy-proven rejections in the control group. Two patients in the biologic-treated group developed skin cancer (basal cell carcinoma) and one patient had renal cell carcinoma vs. two cases in the control group (basal cell carcinoma and prostate cancer). There were two deaths attributed to COVID-19 infection and a car accident compared to none in the control group (Figure 2).
During the follow-up period, there were 6 cases of biologic treatment discontinuation, mainly due to loss of efficacy. One patient reinstituted the biologic treatment after the resolution of the infection.
Conclusion: Our real-world cohort demonstrates the feasibility of the use of biologic treatment in SOT recipients with systemic inflammatory diseases. During the follow-up of 5 years, there was an increased risk of infections, that did not preclude the continuation of the biologic therapy. Infection risk should be weighted with the administration of biologics in this highly immunosuppressed group of patients.
To cite this abstract in AMA style:
Furer V, Kersh O, Berman M, Grupper A, Elkayam O. Safety of Biologic Therapy in Kidney Transplant Recipients with Inflammatory Diseases: Real-world Experience from a Tertiary Medical Center [abstract]. Arthritis Rheumatol. 2024; 76 (suppl 9). https://acrabstracts.org/abstract/safety-of-biologic-therapy-in-kidney-transplant-recipients-with-inflammatory-diseases-real-world-experience-from-a-tertiary-medical-center/. Accessed .« Back to ACR Convergence 2024
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-biologic-therapy-in-kidney-transplant-recipients-with-inflammatory-diseases-real-world-experience-from-a-tertiary-medical-center/