Session Information
Session Type: Abstract Submissions
Session Time: 4:45PM-5:15PM
Background/Purpose:
JIA is the most common chronic inflammatory rheumatic disease of childhood. Due to their known safety and efficacy, TNF inhibitors are used for long-term control of pJIA disease. The purpose of this analysis was to evaluate the 7 year (y) safety of Adalimumab treatment with or without methotrexate (ADA±MTX) when used in current clinical practice for treatment of patients (pts) with active pJIA.
Methods:
This is a 7 y interim analysis of an ongoing, multicenter, non-interventional, observational registry of pts with pJIA with up to10 y safety follow-up. Included pts were treated with either ADA±MTX or MTX alone as comparison arm according to routine clinical care in US, EU, and Australia. MedDRA observational adverse events (AEs) were recorded from 1st day in the registry through last contact, irrespective of the duration of registry treatment.
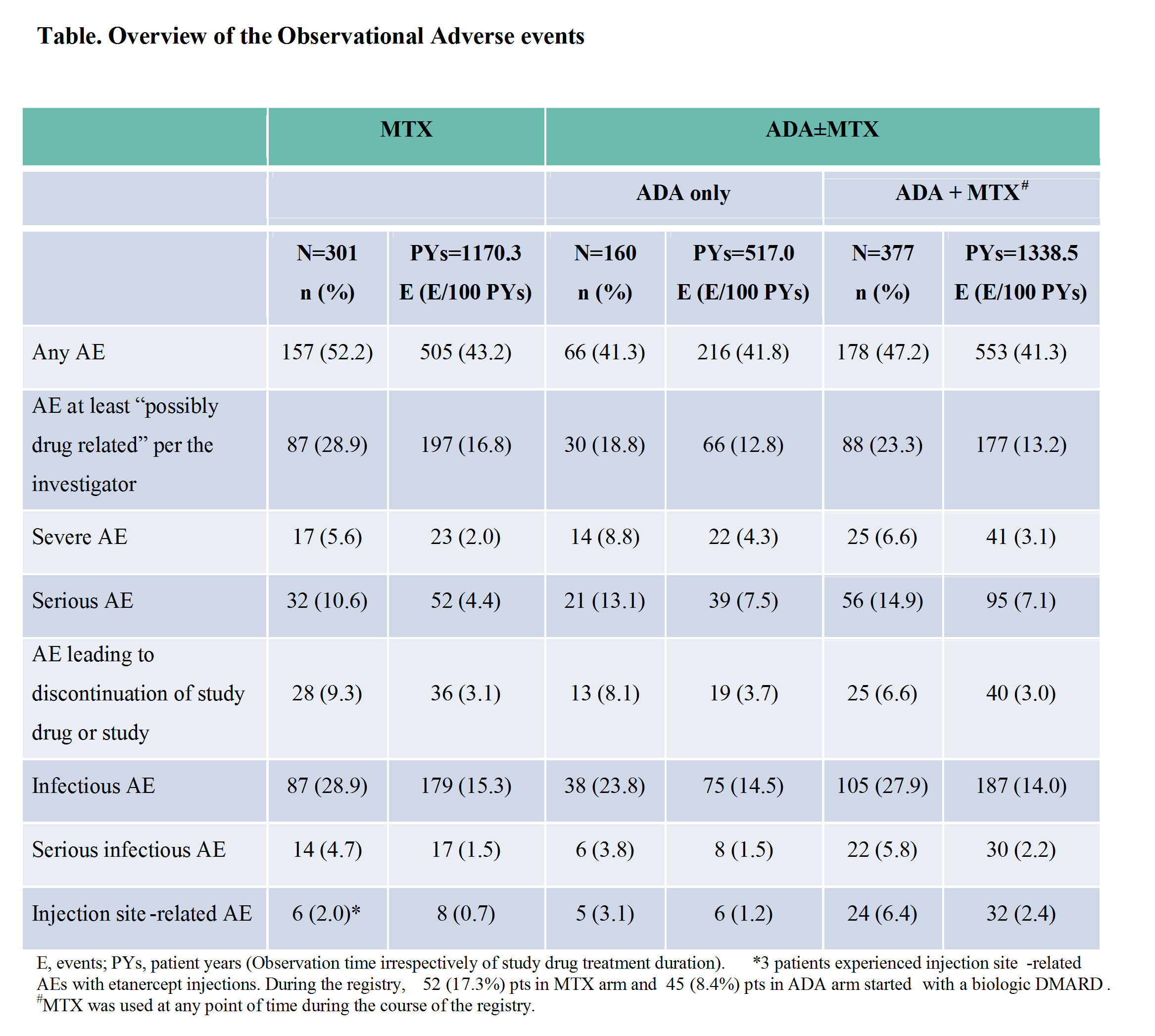
Results:
In January 2014, enrollment was complete. As of June 1, 2016 cut-off date, 838 pts (301- MTX arm and 537 – ADA±MTX arm) were treated in the registry. There were 39 pts who rolled over from MTX to the ADA±MTX arm. At registry entry mean pJIA disease duration was 1.3 y and 3.7 y and mean AJC71 was 5.8 and 5.2 for MTX and ADA±MTX arms, respectively. CHAQ disability index was 0.6 for both arms. The mean duration of study drug exposure in registry was 2.0 y (range: 0.0 – 7.1) and 2.5 y (range: 0.0 – 7.9) for MTX and ADA±MTX arms, respectively. The mean duration of observation in registry was 3.9 y (range: 0.0 – 7.2) and 3.5 y (range: 0.0 – 7.9) for MTX and ADA±MTX arms, respectively. Overall, 213 pts (70.8%) in MTX and 225 pts (41.9%) in ADA±MTX arms discontinued registry drug through 7 y. The main reasons for registry drug discontinuation for MTX arm: pts required additional therapy (32.6%), other (13.3%), lack of efficacy (11.6%), AEs (9.3%), or pts achieved JIA remission (8.6%), and for ADA±MTX arm: lack of efficacy (17.9%), other (7.3%), lost to follow-up (5.6%), AEs (5.4%), or pts achieved JIA remission (5.0%). Frequencies and rates of treatment-emergent AEs (from 1st dose date of registry drug in registry up to last dose + 70 days in registry, excluding AEs occurring during treatment interruption) were similar to those reported for observational AEs (from 1st day in registry up to last contact irrespective of drug treatment duration) (Table). The rate of serious infections was similar between MTX and ADA±MTX arms. One pt (0.2%) reported an event of opportunistic infection (fungal oesophagitis) in ADA±MTX arm. No reports of deaths, malignancies, active tuberculosis, oral candidiasis, demyelination, or congestive heart failure. Data on safety and effectiveness of ADA±MTX background therapy compared to MTX alone will be presented, that adjusts for differences in treatment durations.
Conclusion:
Overall, ADA±MTX was well-tolerated in these pts with pJIA with no new safety signals. The retention rate for registry drug was higher in ADA±MTX arm compared to MTX arm.
To cite this abstract in AMA style:
Brunner H, Ruperto N, Nanda K, Toth M, Foeldvari I, Bohnsack JF, Milojevic D, Rabinovich CE, Kingsbury D, Marzan K, Quartier P, Minden K, Chalom E, Horneff G, Kuester RM, Dare JA, Bereswill M, Kalabic J, Kupper H, Lovell DJ, Martini A. Safety of Adalimumab ± Methotrexate for the Treatment of Polyarticular Juvenile Idiopathic Arthritis (pJIA): STRIVE Registry [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 4). https://acrabstracts.org/abstract/safety-of-adalimumab-%c2%b1-methotrexate-for-the-treatment-of-polyarticular-juvenile-idiopathic-arthritis-pjia-strive-registry/. Accessed .« Back to 2017 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-of-adalimumab-%c2%b1-methotrexate-for-the-treatment-of-polyarticular-juvenile-idiopathic-arthritis-pjia-strive-registry/