Session Information
Date: Tuesday, November 10, 2015
Title: Systemic Sclerosis, Fibrosing Syndromes, and Raynaud's - Clinical Aspects and Therapeutics II
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Interstitial lung disease (ILD) is a common and serious complication
of systemic sclerosis (SSc). Pirfenidone, a novel antifibrotic agent, has been shown
to be safe and effective in the treatment of idiopathic pulmonary fibrosis
(IPF). The LOTUSS study was designed to assess the safety and tolerability of
pirfenidone in patients with SSc-ILD.
Methods: This
is an open-label, 16-week study. Patients were randomized to a 2- or 4-week
titration to the target dose of 2403 mg/day. Eligibility required a diagnosis
of SSc ≤7 years from first non-Raynaud’s symptom, HRCT-confirmed ILD, FVC
≥50% and DLco ≥40%, absence of clinically significant
pulmonary hypertension or severe GERD. Stable treatment with mycophenolate mofetil
(MMF) or oral cyclophosphamide was permitted. Safety assessments included collection
of treatment emergent adverse events (TEAEs), vital signs, ECGs and laboratory
tests. Though the study was not designed or powered to evaluate efficacy, FVC
%-predicted, DLco %-predicted, modified Rodnan skin score (mRSS), Mahler
BDI/TDI, and UCLA SCTC GIT 2.0 were recorded at baseline and 4 months.
Results: Of
the 63 patients enrolled, the mean (SD) age was 50.6 (12.3) years; the majority
were female (82.5%) and white (76.2%). The mean (SD) SSc duration was 38.3 (26.0)
months. Forty patients (63.5%) were on MMF and the rest (36.5%) were not receiving
any immunosuppressants. The mean (SD) mRSS, %FVC and %DLco at
baseline were 11.4 (9.6), 76.0 (14.2) and 59.7 (16.5), respectively.
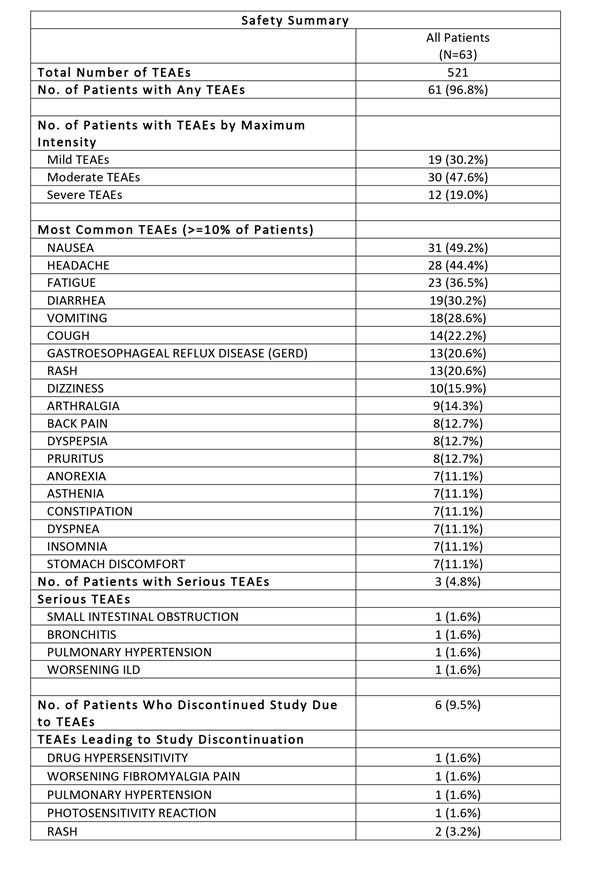
The frequency and type of TEAEs were
similar for both titration groups. The safety results are summarized below. No
clinically significant changes in vital signs, ECGs, or laboratory tests were observed.
At week 16, the median change from baseline
in %FVC was -0.5% (range -42% to 12%); 10 patients (16.7%) had an increase
≥5% whereas 5 (8.3%) had a decrease >5% at week 16. Median change
from baseline in %DLco was 1.5% (range -24.0% to 40.0%); 19 subjects
(31.7%) had an increase ≥5% vs. 10 (16.7%) had a decrease >5% at week
16. Minor changes (mean±SD) were observed in Mahler TDI (1.0±3.41) and mRSS
(-0.4±3.71). No change was noted in the GI symptoms on UCLA SCTC GIT 2.0.
Conclusion:
In the 16-week, open-label trial of pirfenidone in SSc-ILD, pirfenidone was
safe and generally well-tolerated in SSc-ILD patients, despite pre-existing
co-morbidities, including underlying GI disease, and concomitant use of MMF.
The AEs were expected and consistent with those previously seen in IPF trials.
The data support further investigation of pirfenidone in SSc-ILD.
To cite this abstract in AMA style:
Khanna D, Albera C, Fischer A, Seibold JR, Khalidi NA, Raghu G, Chung L, Schiopu E, Chen D, Gorina E. Safety and Tolerability of Pirfenidone in Patients with Systemic Sclerosis Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/safety-and-tolerability-of-pirfenidone-in-patients-with-systemic-sclerosis-interstitial-lung-disease/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-tolerability-of-pirfenidone-in-patients-with-systemic-sclerosis-interstitial-lung-disease/