Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Lenabasum is a synthetic, non-immunosuppressive, selective cannabinoid receptor type 2 agonist that activates resolution of innate immune responses. Lenabasum had acceptable safety and tolerability, and improved multiple efficacy outcomes in the double-blinded, randomized, placebo-controlled Part A of Phase 2 trial JBT101-SSc-001 (NCT02465437) in diffuse cutaneous SSc (dcSSc) subjects.
Objective: To provide long-term open-label safety and efficacy data in dcSSc subjects in study JBT101-SSc-001.
Methods: Subjects who completed Part A were eligible to receive oral lenabasum 20 mg BID in an open-label extension (OLE) that assessed safety and efficacy at 4 weeks, then every 8 weeks.
Results: 36/38 (95%) eligible subjects enrolled in the OLE, with mean interval of 134 (range 33-392) days or 19.1 weeks from end of dosing in Part A to start of OLE when subjects received only standard-of care drugs. 34/36 (94%) subjects were on stable doses of immunosuppressive drugs. At the time of data cut-off, 5/36 (13.9%) subjects had discontinued the OLE for reasons all unrelated to lenabasum: difficulty coming for study visits (n = 2), fatigue, inflamed tendons, and high dose steroid-induced scleroderma renal crisis. Of the remaining 31 subjects, 27 (87.1%) had already completed ≥ 1 year of dosing in OLE. Adverse events (AEs, n = 180) occurred in 33/36 (91.7%) subjects, with 7/36 (19.4%) subjects having ≥ 1 AE related to lenabasum. No subject had a serious or severe AE related to lenabasum. Three serious AEs occurred: renal crisis, thumb fracture, and digital ulcer. AEs that occurred in ≥ 10% of subjects (n, % of subjects) were: upper respiratory tract infection (8, 22.2%), skin ulcer, arthralgias, urinary tract infection (5, 13.9% each), and diarrhea (4, 11.1%). Mild dizziness occurred in 3 (8.3%) subjects.
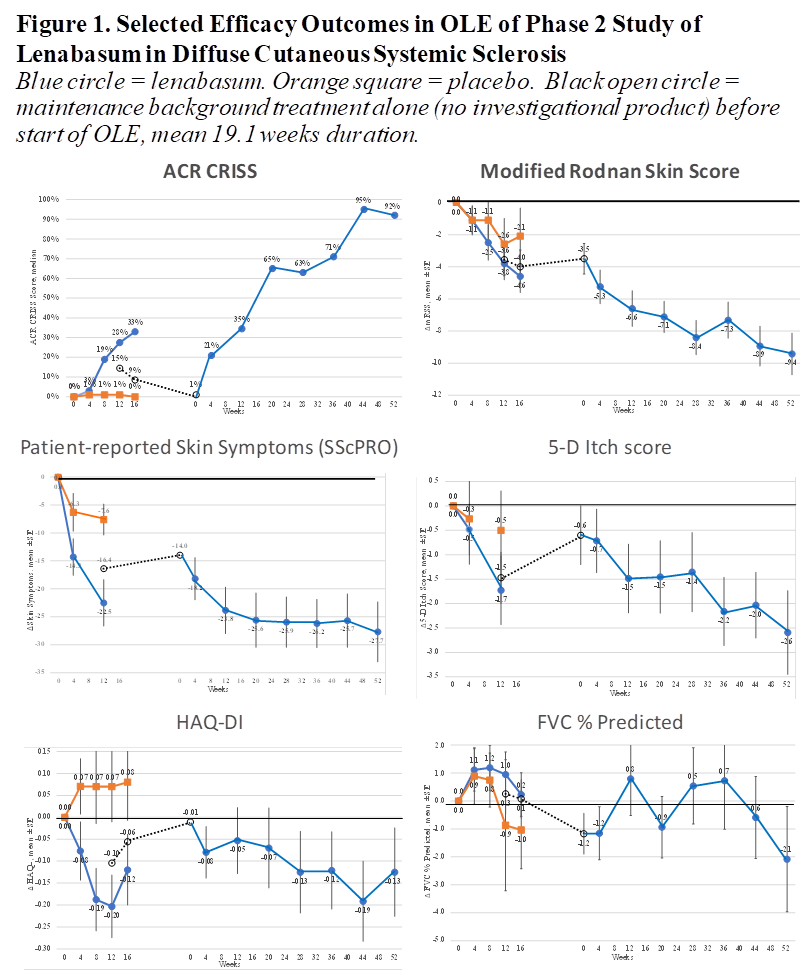
Improvement was seen in multiple physician- and patient-reported efficacy outcomes compared to study start and start of OLE (examples in Figure 1), including Combined Response Index in diffuse cutaneous Systemic Sclerosis (CRISS), mRSS, HAQ-DI, Physician Global Assessment, skin symptoms, itch, and multiple PROMIS-29 domains. FVC % predicted was relatively stable. Compared to baseline at study start, the CRISS median score was 92% (23%, 100% IQR) at Week 52 and mRSS declined by mean (SD) = 9.4 (8.43) and 41.3% (32.7%) from baseline, with 35% of subjects newly achieving a low mRSS ≤ 10.
Conclusion: In OLE of Phase 2 trial JBT101-SSc-001, lenabasum continues to have acceptable safety and tolerability in dcSSc with no severe or serious AEs or study discontinuations related to lenabasum. Only about 1 in 5 subjects had an AE related to lenabasum over 1-year OLE dosing. ACR CRISS score, mRSS, Physician Global Assessment, and multiple patient-reported outcomes show continued improvement, although background therapy, potential for spontaneous improvement, and open-label dosing limit what can be definitely attributed to lenabasum.
To cite this abstract in AMA style:
Spiera RF, Hummers LK, Chung L, Frech TM, Domsic RT, Hsu V, Furst DE, Gordon JK, Mayes MD, Simms RW, Lee E, Constantine S, White B. Safety and Efficacy of Lenabasum in an Open-Label Extension of a Phase 2 Study in Diffuse Cutaneous Systemic Sclerosis Subjects [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/safety-and-efficacy-of-lenabasum-in-an-open-label-extension-of-a-phase-2-study-in-diffuse-cutaneous-systemic-sclerosis-subjects/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-lenabasum-in-an-open-label-extension-of-a-phase-2-study-in-diffuse-cutaneous-systemic-sclerosis-subjects/