Session Information
Date: Sunday, November 13, 2022
Title: Abstracts: Spondyloarthritis Including PsA – Treatment II: PsA
Session Type: Abstract Session
Session Time: 3:00PM-4:30PM
Background/Purpose: Deucravacitinib (DEUC) is a novel, oral, selective, allosteric inhibitor of tyrosine kinase 2 (TYK2) that acts by binding to the unique TYK2 regulatory domain, thereby suppressing signaling of key cytokines (eg, IL-23) involved in skin psoriasis and PsA pathogenesis. Results from the initial 16-week, placebo (PBO)-controlled period (Part A) of a 52-week, blinded phase 2 trial in PsA showed that DEUC was significantly more efficacious than PBO.1 The Psoriatic Arthritis Disease Activity Score (PASDAS), a validated comprehensive measure assessing a variety of PsA clinical domains, was used to assess efficacy of DEUC up to 52 weeks. The objective of this study was to evaluate the safety and efficacy of DEUC in Part B (weeks 16-52) in the phase 2 PsA trial.
Methods: Patients with PsA were randomized 1:1:1 to PBO, DEUC 6 mg once daily (QD), or 12 mg QD. After week 16 (Part A), patients could enroll in an optional, double-blind period until week 52 (Part B). In Part B, patients receiving DEUC who had achieved minimal disease activity (MDA) at week 16 continued DEUC treatment and those who had not achieved MDA were switched to ustekinumab (UST) at the approved PsA dose. All patients treated with PBO in Part A switched to UST in Part B. Patients were assessed up to 52 weeks for adverse events (AEs) and exploratory efficacy endpoints including change in PASDAS. Analyses were descriptive using data as observed.
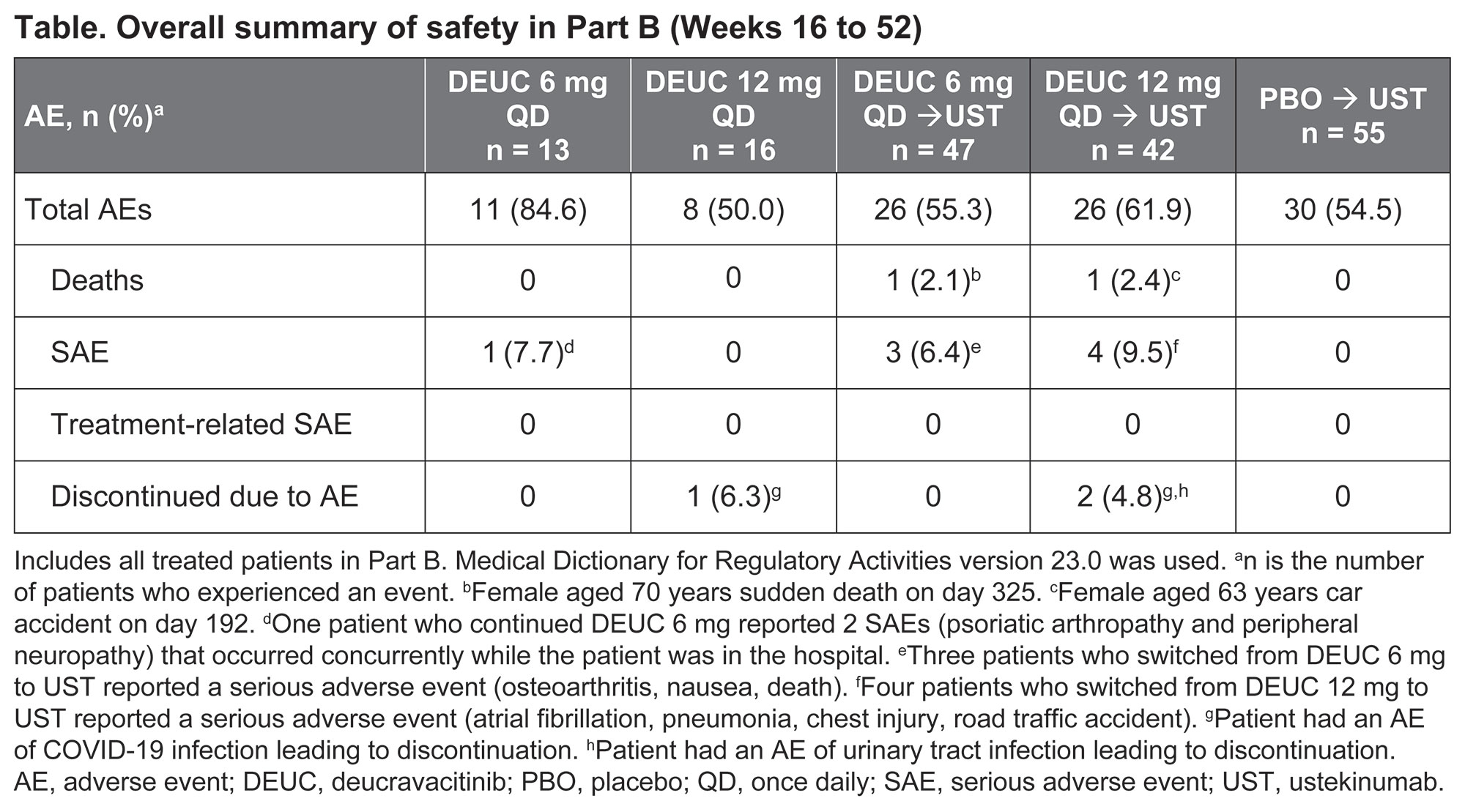
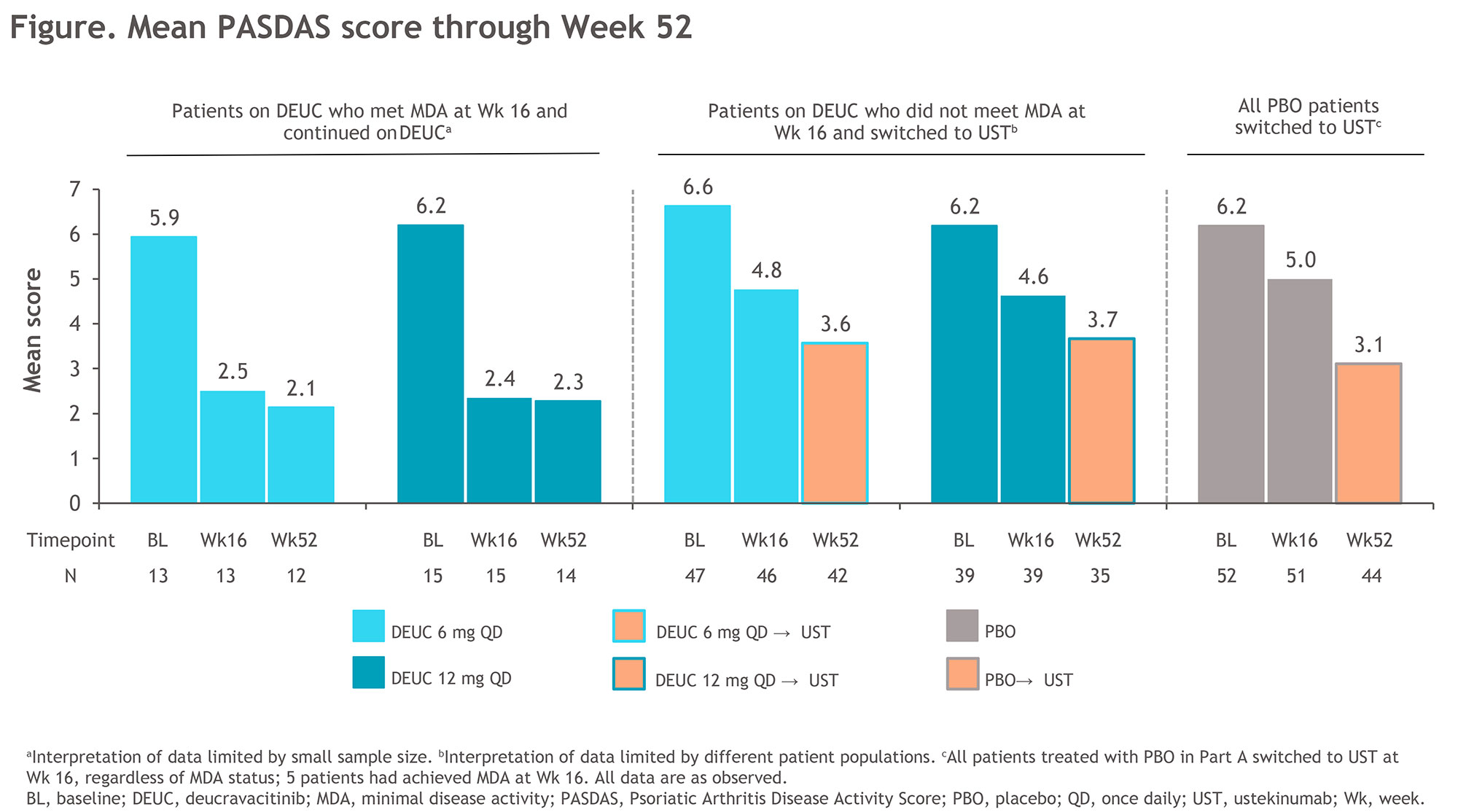
Results: Of 203 patients randomized in Part A, 180 (89%) completed 16 weeks of treatment and 173 (96%) of these patients chose to enroll in Part B. Of 118 patients initially randomized to DEUC, 25% (29/118; 6 mg QD, 22% [13/60]; 12 mg QD, 28% [16/58]) achieved MDA at week 16 and continued at the same dose. All other patients switched to UST in Part B: PBO, 100% (55/55; including 5 patients who had achieved MDA at week 16); DEUC 6 mg QD, 78% (47/60); DEUC 12 mg QD, 72% (42/58). The safety profile of DEUC in Part B (Table) was consistent with that in Part A, and all AEs were mild or moderate except 2 AEs in 1 patient with severe cataract/macular fibrosis. There were no opportunistic infections, herpes zoster, malignancy, thrombotic events, or treatment-related serious AEs reported in patients who remained on DEUC. Decreases in mean PASDAS score observed at week 16 were maintained at week 52 in patients who continued on DEUC (Figure). Improvements in other clinical and patient-reported outcomes, including Disease Activity Index for Psoriatic Arthritis, Psoriasis Area and Severity Index, Functional Assessment of Chronic Illness Therapy-Fatigue, and Patient Global Assessment of Disease Activity, were also sustained at week 52 in patients who continued DEUC treatment. Patients who had not achieved MDA on DEUC at week 16 showed a decrease in mean PASDAS score at week 52 after switching to UST.
Conclusion: In the 16- to 52-week blinded Part B of a phase 2 study in patients with PsA, no new safety signals were observed with continuous DEUC treatment vs the earlier Part A period. Efficacy in PASDAS, as well as other key efficacy measures, was maintained with continued DEUC treatment through week 52.
Reference
1. Mease PJ, et al. Efficacy and Safety of Selective TYK2 Inhibitor, Deucravacitinib, in a Phase 2 Trial in Psoriatic Arthritis. Ann Rheum Dis. 2022;81:815-822.
To cite this abstract in AMA style:
Mease P, Deodhar A, van der Heijde D, Behrens F, Kivitz A, Neal J, Nys M, Lehman T, Delev N, Korish S, Nowak M, Banerjee S. Safety and Efficacy of Deucravacitinib, an Oral, Selective Tyrosine Kinase 2 Inhibitor, in Patients with Psoriatic Arthritis: 52-Week Results from a Randomized Phase 2 Trial [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/safety-and-efficacy-of-deucravacitinib-an-oral-selective-tyrosine-kinase-2-inhibitor-in-patients-with-psoriatic-arthritis-52-week-results-from-a-randomized-phase-2-trial/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-deucravacitinib-an-oral-selective-tyrosine-kinase-2-inhibitor-in-patients-with-psoriatic-arthritis-52-week-results-from-a-randomized-phase-2-trial/