Session Information
Date: Monday, November 6, 2017
Title: Osteoporosis and Metabolic Bone Disease – Clinical Aspects and Pathogenesis
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: As renal function may decline with age, it is important to understand the safety and efficacy of therapeutic agents for postmenopausal osteoporosis (PMO) and the effect these agents may have on intrinsic renal function in patients with age-related renal insufficiency. We assessed the safety and efficacy of denosumab among subjects with mild-to-moderate renal insufficiency who participated in the “Fracture REduction Evaluation of Denosumab in Osteoporosis every 6 Months” (FREEDOM) extension study.
Methods: Subjects were grouped based on baseline estimated glomerular filtration rate (eGFR) normalized to body surface area and calculated using the MDRD study equation. Change in eGFR from baseline to last on-study visit for each subject was assessed and summarized. The annualized rates of new vertebral fractures (VFx), non-VFx, and adverse events (AEs) were assessed for the subgroups of subjects with normal renal function (eGFR ≥ 90 mL/min), mild renal insufficiency (eGFR = 60-89 mL/min; CKD stage 2), or moderate renal insufficiency (eGFR = 30-59 mL/min; CKD stage 3). These outcomes were evaluated for the long-term arm (up to 10 years of denosumab treatment) and the crossover arm (up to 7 years of denosumab treatment).
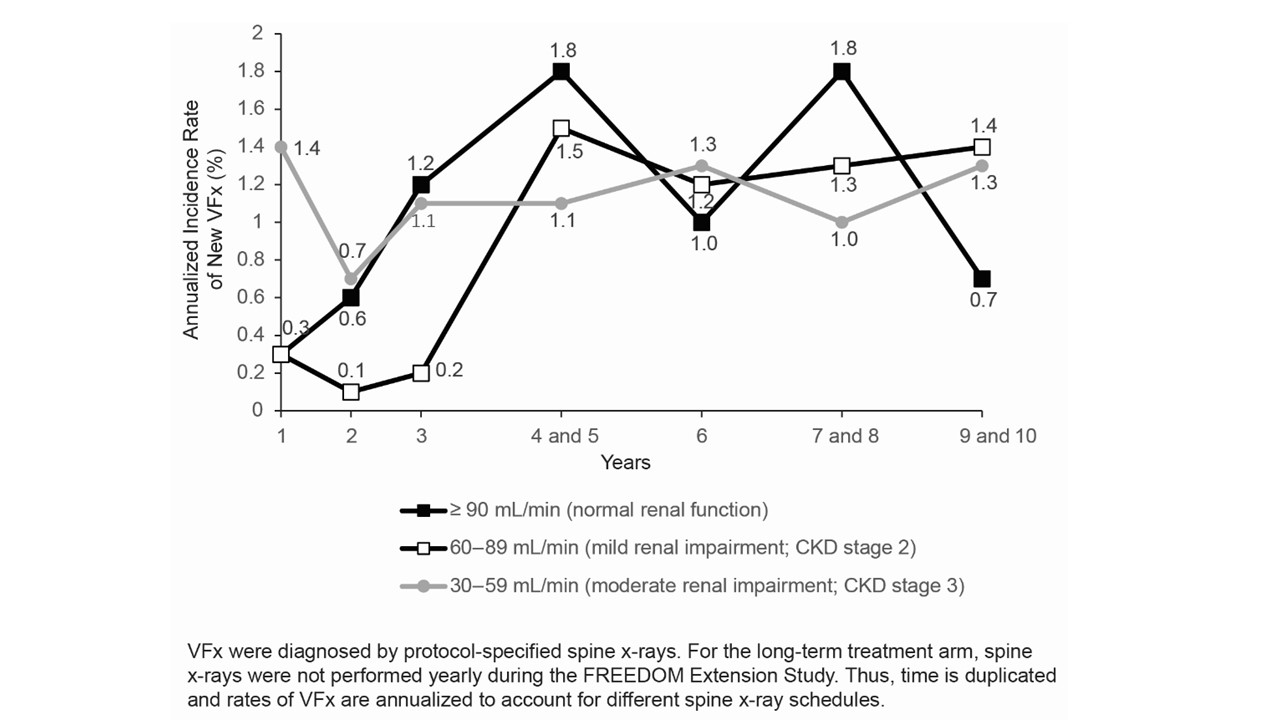
Results: The majority of subjects in the long-term arm (1969/2343; 84%) and crossover arm (1781/2206; 81%) had mild or moderate renal insufficiency (CKD stage 2 or 3) prior to receiving denosumab. Few subjects (n = 4 long-term arm; n = 5 crossover arm) had an eGFR = 15-29 mL/min (CKD stage 4) at baseline, and none had CKD stage 5. Most subjects (1325/1969 [67%] long-term arm; 1216/1781 [68%] crossover arm) with baseline CKD stage 2 or 3 had relatively stable renal function, remaining within the same CKD subgroup at the last on-study visit. Less than 1% of subjects progressed from CKD stage 2 or 3 to CKD stage 4, and no subjects initiated renal replacement therapy. The incidence of new VFx was similar among subjects with normal eGFR or CKD stage 2 or 3 in both the long-term arm (Figure) and crossover arm (data not shown). The percentage of subjects reporting serious AEs was similar among the renal subgroups for both the long-term arm (54% normal GFR; 52% CKD stage 2; 57% CKD stage 3) and crossover arm (43% normal GFR; 42% CKD stage 2; 45% CKD stage 3).
Conclusion: The safety and efficacy of denosumab did not substantially differ among subjects with mild-to-moderate renal insufficiency. Furthermore, long-term exposure to denosumab does not appear to have a meaningful effect on renal function in women with PMO.
Figure. Annualized Incidence Rate of New VFx for the Long-Term Treatment Arm
To cite this abstract in AMA style:
Broadwell A, Ebeling PR, Franek E, Goemaere S, Wagman RB, Yin X, Yue S, Miller PD. Safety and Efficacy of Denosumab Among Subjects with Mild-to-Moderate Chronic Kidney Disease (CKD) in the “Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months” Extension Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/safety-and-efficacy-of-denosumab-among-subjects-with-mild-to-moderate-chronic-kidney-disease-ckd-in-the-fracture-reduction-evaluation-of-denosumab-in-osteoporosis-every-6-months-ex/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-denosumab-among-subjects-with-mild-to-moderate-chronic-kidney-disease-ckd-in-the-fracture-reduction-evaluation-of-denosumab-in-osteoporosis-every-6-months-ex/