Session Information
Date: Saturday, November 6, 2021
Title: Abstracts: Muscle Biology, Myositis & Myopathies (0441–0444)
Session Type: Abstract Session
Session Time: 9:30AM-9:45AM
Background/Purpose: Treatment of Idiopathic Inflammatory Myositis (IIM) includes steroids and immunosuppressive agents. Targeting IL6, IL1, TNF for treatment of IIM has not been successful, and the role of B-cell depleting therapy remains uncertain. This study was designed to assess the safety and efficacy of belimumab for IIM patients.
Methods: We conducted a 40-week multicenter randomized, double blind, placebo controlled clinical trial with a 24-week open label extension of intravenous (IV) belimumab for adult patients with refractory IIM. All patients met Peter and Bohan criteria and ACR 2017 classification criteria of polymyositis / dermatomyositis (PM/DM) with PM diagnosis adjudication. Refractory IIM was defined as inadequate response/ intolerance to 3 months of glucocorticoids and/or at least one immunosuppressive agent (IS). Standard Core Set Measures (CSM) with MMT8 < 125/150 were used to define active disease. Patients on standard of care (SoC) therapy were randomized 1:1 to IV belimumab 10mg/kg or placebo for 40 weeks followed by 24 weeks belimumab 10mg/kg in open label phase. The primary outcomes were the proportions of patients reaching a Definition of Improvement (DOI) and Total Improvement Score (TIS) at week 40 in belimumab arm vs. SoC alone arm. Secondary endpoints included DOI, TIS at week 64, CSM changes, prednisone dose change, safety profiles at 40 and 64 weeks. Descriptive statistics (means, SDs, medians, ranges, frequencies, proportions) are presented. The Mann-Whitney test and Fisher’s exact test were used to analyze continuous and categorical variables, respectively.
Results: 16 patients were randomized, 15 received at least 4 doses of belimumab or placebo to be included in the analysis (9 belimumab; 6 placebo) (Baseline characteristics are in Table 1).
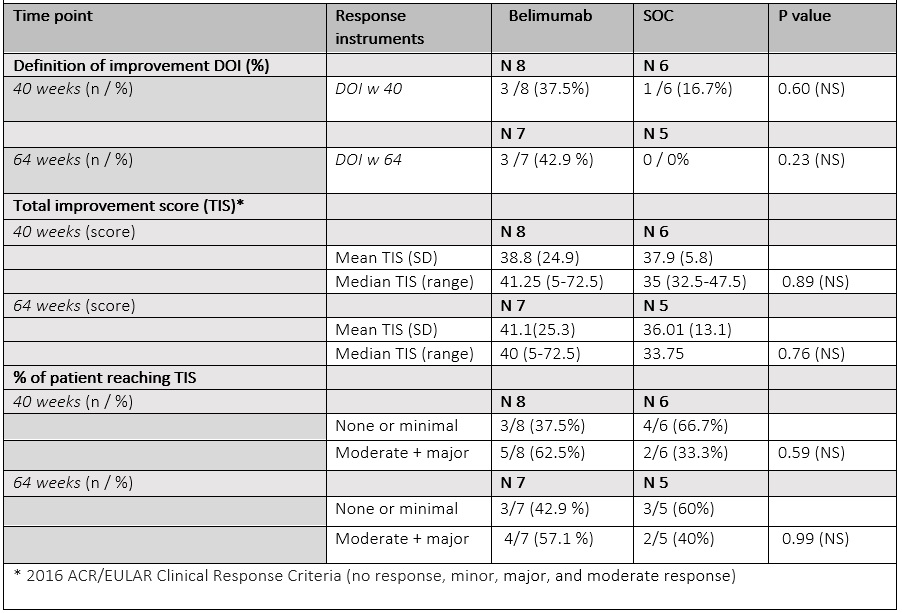
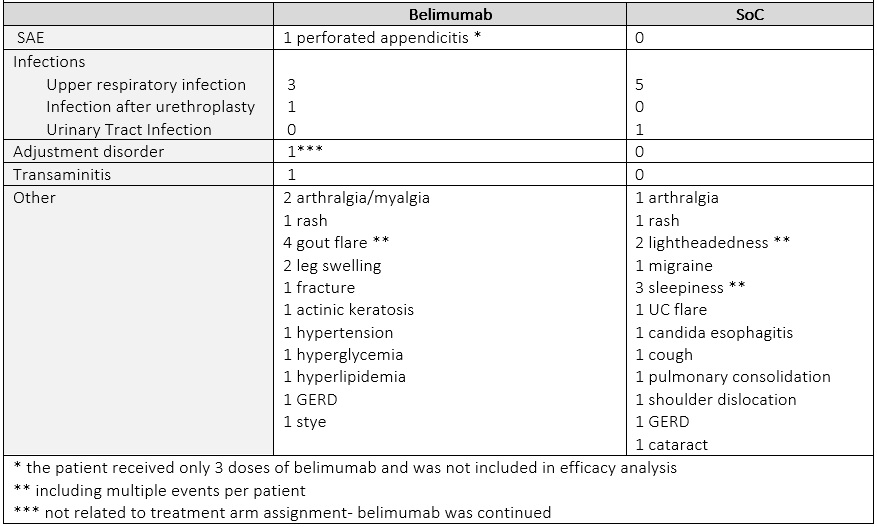
The proportion of patients reaching DOI by week 40 was numerically higher in belimumab arm (belimumab 37.5% /SoC 16.7 %). The mean TIS was similar between 2 groups (mean (SD) belimumab 38.8 (24.9)/ SoC 37.9 (5.8). At week 64, 42.9 % of patients on belimumab achieved DOI, while no patients in the original SoC arm did. Numerically more patients on belimumab had moderate or major improvement than on SoC (belimumab 62.5%/ SoC 33.3%) at week 40, and (belimumab 57.1% /SoC 40.0 %) at week 64. Of 15 patients only 2 had a major response at week 40 (TIS= 72.5) that sustained to week 64; both had received belimumab. Infections were evenly distributed between two arms. One patient in the belimumab arm had an adjustment disorder exacerbation, determined not to be belimumab related and the drug was continued with no interruption (Table 3).
Conclusion: In this 40-week multicenter randomized, double blind placebo-controlled trial with 24 weeks open label phase, we observed numerically higher proportion of patients on belimumab reaching DOI vs. on SoC only arm. A higher proportion of patients on Belimumab achieved sustained moderate or major TIS at 40 and 64 weeks compared to SOC. Detected differences were not statistically significant, however the sample size was small.
To cite this abstract in AMA style:
Chadha P, Narain S, Nandkumar P, Sison C, Schiopu E, Levine T, Tsang J, Marder G. Safety and Efficacy of Belimumab in the Treatment of Adult Idiopathic Inflammatory Myositis (Polymyositis and Dermatomyositis) [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/safety-and-efficacy-of-belimumab-in-the-treatment-of-adult-idiopathic-inflammatory-myositis-polymyositis-and-dermatomyositis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-belimumab-in-the-treatment-of-adult-idiopathic-inflammatory-myositis-polymyositis-and-dermatomyositis/