Session Information
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Since inhibition of either Tumor Necrosis Factor (TNF) or interleukin 17 (IL-17) alone has demonstrated efficacy in psoriatic arthritis (PsA) on both joint and skin features, dual inhibition of TNF and IL-17 holds the promise for a superior therapeutic profile. ABT-122 is a dual variable domain immunoglobulin (DVD-Ig™) inhibitor of TNF and IL-17A that has shown an enabling safety profile from phase 1 studies. The purpose of this study was to investigate the safety and efficacy of ABT-122 in PsA patients (pts) with inadequate response to methotrexate (MTX).
Methods: Pts with active PsA (N=240) on background MTX were randomized in a 3:3:3:1 ratio to receive ABT-122 (120 mg every week [ew], or 240 mg ew), adalimumab (ADA) 40 mg every other week (eow), or matching placebo (PBO) subcutaneously in a 12-week (wk) double-blind, parallel group study. The primary efficacy endpoint was the ACR20 responses versus PBO at week (wk) 12. Additional efficacy endpoints included ACR50/70, low disease activity (LDA) and clinical remission (CR) based on DAS28 (hsCRP), and PASI 75/90 responses in pts who had psoriasis ≥3% body surface area at baseline.
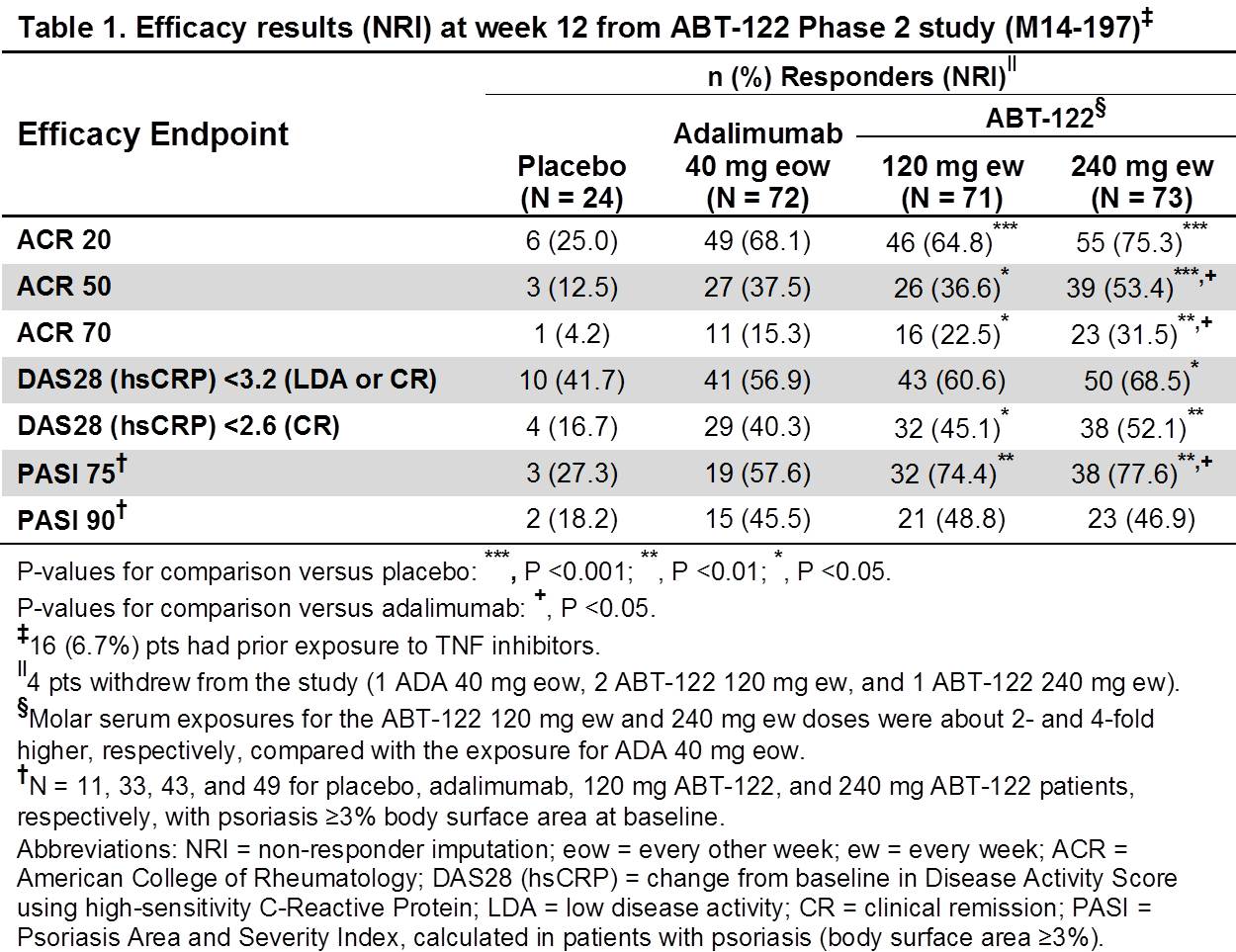
Results: ACR20 responses for both ABT-122 dose groups were statistically superior to PBO and comparable to ADA (Table 1). ACR50 and ACR70 responses were superior to PBO for all active dose arms, with numerical superiority for ABT-122 240 mg dose versus ADA. LDA or CR rates based on DAS28 (hsCRP) were superior to PBO across all active dose arms, but did not differ between ABT-122 and ADA. All active dose arms showed higher PASI 75 and PASI 90 response rates than PBO. PASI 75 responses for both ABT-122 doses were numerically greater than ADA; but PASI 90 responses were similar. Treatment-emergent adverse events (TEAEs) were similar across active treatment groups (Table 2), with no differences in serious AEs (SAEs) or discontinuations; infection rates were higher in active treatment groups than PBO, however no serious infections were reported in any treatment group. There were no reports of drug-related SAEs, systemic hypersensitivity reactions, severe injection site reactions, or dose-limiting or clinically-concerning laboratory abnormalities in any dose arms.
Conclusion: Efficacy of ABT-122 over 12 wks was superior to PBO on all clinical outcomes. ACR20 and PASI 90 responses for both ABT-122 doses were similar to ADA, but ACR50/70 and PASI 75 responses for 240 mg ABT-122 were numerically superior versus ADA. ABT-122 had an acceptable safety profile in PsA pts on background MTX. Dual neutralization of TNF and IL-17 with a DVD-Ig offers similar infection rates to ADA, but does not have differentiated efficacy over 12 wks in PsA pts.
To cite this abstract in AMA style:
Mease PJ, Genovese MC, Weinblatt M, Peloso PM, Chen K, Li Y, Mansikka HT, Khatri A, Othman AA, Wishart N, Liu J, Padley RJ. Safety and Efficacy of ABT-122, a TNF and IL-17–Targeted Dual Variable Domain (DVD)–Ig™, in Psoriatic Arthritis Patients with Inadequate Response to Methotrexate: Results from a Phase 2 Trial [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/safety-and-efficacy-of-abt-122-a-tnf-and-il-17-targeted-dual-variable-domain-dvd-ig-in-psoriatic-arthritis-patients-with-inadequate-response-to-methotrexate-results-from/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-efficacy-of-abt-122-a-tnf-and-il-17-targeted-dual-variable-domain-dvd-ig-in-psoriatic-arthritis-patients-with-inadequate-response-to-methotrexate-results-from/