Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: The use of hydroxychloroquine (HCQ) has long been established in Systemic Lupus Erythematosus
(SLE) and especially as applicable drug during pregnancy. Recently, beneficial effects and safety of HCQ have been re-discussed in the light of a change in the summary of product characteristics in some countries. More current studies are required to provide patients with evidence-based advice regarding this essential drug when counselling for pregnancy.
We therefore sought to examine the impact of HCQ on pregnancy outcomes of SLE women in a real-world cohort.
Methods: Pregnancies of women with SLE from an outpatient pregnancy clinic were prospectively evaluated before and throughout gestation. Maternal and fetal outcomes in women without HCQ therapy (group A) were compared to pregnancies under HCQ treatment from 1st trimester on (group B). A multiple logistic regression was performed with adjustment for confounding factors.
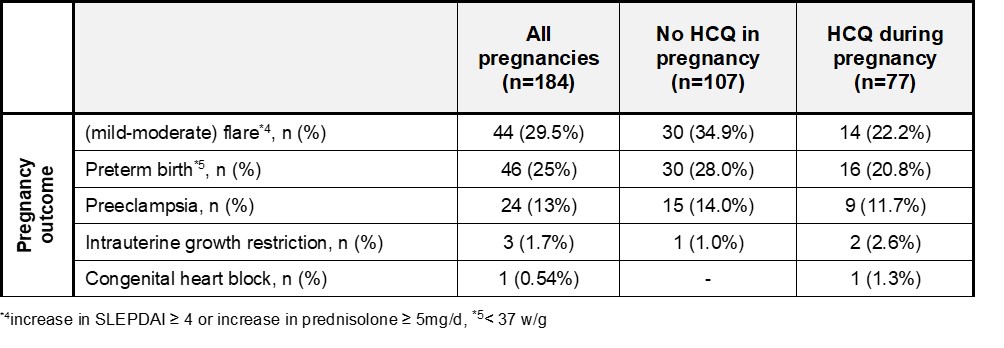
Results: We enrolled 184 live births from singleton pregnancies in 145 women (n=77 with HCQ and n=107 w/o HCQ). One neonatal death (group B) occurred after severe preeclampsia at 24 weeks of gestation (w/g) linked to noncompliance in a woman with high-risk aPL profile. One child (group B) was born with mycophenolate mofetil embryopathy.
Women in the HCQ group had a significantly lower rate of preterm births [aOR 0.31 (95%-CI: 0.15-
0.64), p = 0.026]. Regarding preeclampsia, we found a tendency towards less incidence with the use of HCQ [aOR 0.49 (95%-CI: 0.23-1.03), p = 0.24]. These improved outcomes are opposed by a higher frequency of risk factors in group B (lupus nephritis, high-risk aPL profile, slightly more hypertension) and a tendency towards more severe SLE (expressed in terms of increased use of Azathioprine) (Table 1). Nevertheless, women with HCQ therapy experienced significantly less flares during pregnancy [aOR 0.18 (95%-CI: 0.09-0.38), p = 0.013].
Conclusion: Compared to pregnancies without HCQ, those with HCQ showed significantly lower rates of flares and preterm births and tended to have fewer pre-eclampsia. Like other studies on HCQ in lupus pregnancy, this one is influenced by the indication and adherence. However, since the safety of HCQ is confirmed, it is in line with current recommendations to continue HCQ throughout pregnancy.
 Table 1: Patient characteristics
Table 1: Patient characteristics
To cite this abstract in AMA style:
Haase I, Schneider M, Brinks R, Fischer-Betz R. Safety and Beneficial Effects of Hydroxychloroquine on Pregnancy Outcomes in Women with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/safety-and-beneficial-effects-of-hydroxychloroquine-on-pregnancy-outcomes-in-women-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-beneficial-effects-of-hydroxychloroquine-on-pregnancy-outcomes-in-women-with-systemic-lupus-erythematosus/