Session Information
Date: Sunday, November 8, 2015
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
PQoL-12 is a validated composite tool (range: 0–120) assessing
patients’ quality of life in PsO and PsA. RAPID3 is another validated composite index (range:
0–10) measuring physical function, pain and patient global assessment in
rheumatoid arthritis and ankylosing spondylitis, but not in PsO/PsA. If RAPID3 could be used to assess PsO/PsA patients’ quality of life, it may save time in busy
practices. This study investigates the correlation between PQoL-12 and RAPID3
in PsO and PsA patients,
and also the cut-off values for RAPID3 that correlate best with PQoL-12 of 48 (mild)
and 96 (moderate QoL impairment).
Methods:
Data from PsO and PsA patients seen from 2008 onwards in the Center of
Excellence in Psoriasis and Psoriatic Arthritis clinic at Oregon Health &
Science University were used (N= 555, 390 with PsO,
165 with PsA). Nonlinear least squares regressions
modeled PQoL-12 with functions of RAPID3 while controlling for time since first
visit. Nonparametric ROC analysis determined RAPID3 scores best correlating
with PQoL-12 cut-offs.
Results:
Baseline mean age was 46.7(12.8) and 45.9(12.8) years for PsO and PsA. The M:F ratio was 3:2 and 1:1 for PsO
and PsA. Mean disease duration was 0.93(1.08) and
1.11(1.20) years for PsO and PsA.
Baseline mean PQoL-12 was 65.8 (29.5) and 75.2 (29.2), and RAPID3 was 2.4 (2.01)
and 3.7 (2.4) for PsO and PsA.
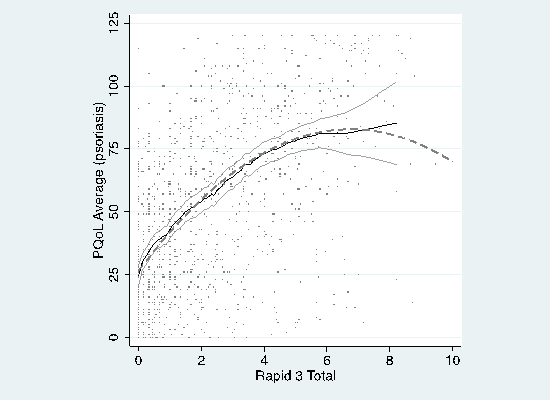
For PsO, PQoL-12 was explained by RAPID3 (b
= 16.6; s.e.= 1.63), RAPID32(b =
-1.22 ; s.e. = 0.0701), time since first visit (b
= -0.00211 ; s.e.= 0.00467), and a saturation (q
= 281; s.e. = 116) of time since first visit2
(b
= 0.000211; s.e.= 0.000174) with adjusted R2=0.414
(figure 1). For PsA, PQoL-12 was explained by RAPID3
(b
=12.9; s.e.= 2.99), change (q = 2.28; s.e.
= 81.5) in slope of RAPID3 (b =-7.39 ; s.e.=3.38
), time since first visit (b = -0.00172 ; s.e.=
0.00685), and a saturation (q = 223; s.e. =
116) of time since first visit2 (b =0.000417; s.e.=
0.000388=4) with adjusted R2=0.340 (figure2). RAPID3 cut-offs for PQoL–12 scores of 48 and 96 in PsO
were 1.38 and 7.22 (71.2% and 88.4% correctly classified) and in PsA were 2.06 and 6.61 (70.4% and 89.0% correctly classified).
Conclusion:
RAPID3 correlates poorly with PQoL-12. These indices assess
different aspects of PsO and PsA,
and provide distinct and important information regarding the patients’ diseases.
Figure 1- Nonparametric
regression of PQoL-12 on RAPID3 in PsO
Figure 2- Nonparametric regression
of PQoL-12 on RAPID3 in PsA
To cite this abstract in AMA style:
Vakil-Gilani K, Dinno A, Garg N, Deodhar AA. Routine Assessment of Patient Index Data 3 Score (RAPID3) and Psoriasis Quality of Life (PQoL-12) Assess Different Domains in Psoriasis (PsO) and Psoriatic Arthritis (PsA) Patients [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/routine-assessment-of-patient-index-data-3-score-rapid3-and-psoriasis-quality-of-life-pqol-12-assess-different-domains-in-psoriasis-pso-and-psoriatic-arthritis-psa-patients/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/routine-assessment-of-patient-index-data-3-score-rapid3-and-psoriasis-quality-of-life-pqol-12-assess-different-domains-in-psoriasis-pso-and-psoriatic-arthritis-psa-patients/